by Cüneyt Köksoy and Umman Sanlidilek
The potential for thrombolysis to improve outcomes for patients with deep venous thrombosis (DVT) seems to be strong. The development of pharmacomechanical thrombolysis techniques has enhanced the ability to efficiently remove large thrombus burden. We report a case series of successful DVT management with pharmacomechanical thrombectomy utilizing a new rotational thrombectomy device.
Methods
Patients with lower limb DVT that underwent pharmacomechanical thrombectomy utilizing Cleaner™ thrombectomy device (Argon Medical Devices, Inc., Plano, TX) were enrolled for the study. Following diagnosis of acute or subacute lower extremity DVT, using popliteal or tibial access, the device was inserted and pharmacomechanical thrombolysis was applied as “single-session” technique. At the end of the procedure patency was confirmed by contrast venography.
Results
Nine patients, 5 males and 4 females, mean age 49.8 (23- 78) years underwent pharmachomechanical thrombolysis for DVT. Three patients had tibio-femoral, five patients had popliteo-iliac venous thrombosis. Duration of symptoms was 17.7 (7-48) days. A temporary vena cava filter was inserted in all patients except one. Access site for thrombolysis was posterior tibial vein in one patient and popliteal vein was in others. Mean amount of tissue-plasminogen activator, a thrombolytic agent, was 19.8 (10-25) mg and duration of procedure was 78.5 (46-120) minutes. All patients except one had complete thrombus resolution at the end of the procedure. There were no complications in terms of pulmonary embolism or bleeding. Symptomatic relief was obtained in every patient, and all patients were discharged next day with anticoagulant therapy.
Conclusions
The Cleaner™ device was an effective method for the treatment of acute DVT. Based on the present data, this device could prove to be a safe and feasible single-session pharmacomechanical thrombolysis (PMT) method for the treatment of acute DVT in broader patient populations and warrants further investigation in large-scale studies.
Background
Although systemic anticoagulation remains the mainstay of (DVT) therapy, thrombolysis or surgical thrombectomy is often indicated in symptomatic patients with severe (DVT). Over the past decade, pharmacomechanical thrombolysis has emerged as an effective treatment modality to open surgical thrombectomy and catheter directed thrombolysis in patients with acute thrombosis.1 Pharmacomechanical thrombolysis may allow for rapid clearance of occlusive thrombus, reducing overall thrombolysis infusion times, doses and length of stay.2-5 Recently, the Society for Vascular Surgery and the American Venous Forum suggest pharmacomechanical strategies over catheter-directed pharmacologic thrombolysis alone if resources are available.
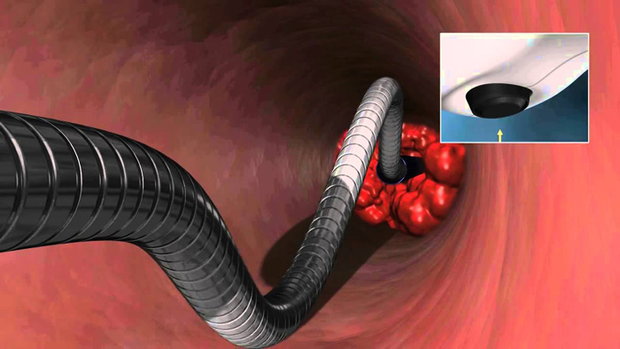
Several pharmacomechanical thrombolysis devices have been developed over the past two decades.7,8 The Cleaner™ thrombectomy device is a percutaneous mechanical thrombectomy catheter which functions by spinning a flexible “S” shaped guidewire atraumatically within the vessel to be treated. This allows the clot to be macerated and aspirated through an introducer sheath. However, information about efficacy of this device is limited.
Objective
The aim of this study was to evaluate the efficacy and safety of pharmacomechanical thrombectomy utilizing a new rotational thrombectomy device for DVT.
Research Design
Over a 7 month period starting from July 2012, 9 patients with symptomatic iliac and femoropopliteal DVT underwent pharmacomechanical thrombolysis with recombinant tissue-plasminogen activator (tPA). In all patients, initial diagnosis of DVT was made by venous duplex imaging. Definitive delineation of the extent of involved venous segments was established by venography.
A temporary inferior vena cava (IVC) filter was placed in before the procedure whenever possible. Then a 6F sheath was placed in the popliteal vein or posterior tibilal vein of the affected side in patients. Ultrasound was used for guidance. Systemic heparinization was initiated by bolus injection of 100 U/kg heparin. A venogram was obtained and a 6F Cleaner™ thrombectomy device was inserted through the introducer sheath. The device was activated and advanced in antegrade fashion. Recombinant form of tissue-plasminogen activator (tPA) Alteplase, (Actilyse, Boehringer Ingelheim, Germany) was delivered through the side port of the device. Following a 2 to 5 minute thrombolysis, the device was deactivated and temporarily withdrawn. Macerated thrombus as well as residual lytic agent was suctioned through the sheath and control venograms were taken to evaluate the treated segments. Then, the device was reinserted and repositioned in the upper segment. Thrombolysis was carried out in segmental fashion. Approximately every 5 cm of the vein was treated using 1 to 2 mg tPA solution for 2 to 5 minutes. This procedure was repeated to the level of the thrombus ends. Finally the device was removed and a completion venogram was performed.
When suboptimal result was obtained, the device was reinserted and activated for one more pass. After the second or third pass, if the control venogram revealed residual thrombus, a 4F infusion catheter (Cragg-McNamara® Valved Infusion Catheter, EV3, Irvine, CA) was placed in and tPA infusion was initiated with 1 mg/kg for 24 hours.
Post-interventional venograms were performed and graded for the quantity of thrombus extraction. Thrombus removal was scored as complete if a venogram showed no further clot remaining after the procedure. Partial thrombus removal indicates those procedures in which any amount of clot remained after the intervention was completed. Following interventional therapy, all patients were continued on subcutaneous low-molecular-weight heparin, all with subsequent conversion to oral warfarin. Whenever possible, temporary IVC filters were removed in one month.
Results
Nine patients, 5 males and 4 females, mean age 49.8 (23- 78) years underwent pharmacomechanical thrombolysis for DVT. Three patients had tibio-femoral, six patients had popliteal-femoral-iliac venous thrombosis. The DVT location was unilateral in all patients (5 left side, 4 right side). Duration of symptoms was 17.7 ± 13.8 (7-48) days.
Patients were taken to the angiography suite within 48 ± 6 hours of presentation. A temporary vena cava filter was inserted in all patients except one. Access site for thrombolysis was posterior tibial vein in one patient and popliteal vein in others. Mean amount of tPA was 19.8 (10-25) mg and duration of procedure was 78.5 (46-120) minutes.
All patients were treated in one session, but one patient required lytic infusion for residual thrombus. At the end of the discharge, successful lysis (>50%) was achieved in 8 (88.9%) of patients. Only one patient with a history of 30 days of iliofemoral thrombosis had >50% residual obstruction in the iliofemoral venous segment on completion venogram despite catheter directed thrombolysis for 24 hours.
There was no complication in terms of pulmonary embolism or bleeding including access site bleeding. Even in the 2 patients with recent surgery, no bleeding occurred. Symptomatic relief was obtained in all patients and patients were discharged next day with anticoagulant therapy.
Venous duplex Ultrasonography demonstrated patent veins and normal valve function in 7 patients at one month. IVC filters were removed in two young patients.
Conclusions
This device can be safely and effectively used for treating acute and subacute DVT in a single session of pharmacomechanical thrombolysis, providing with results of improved functional outcome. The technique has the potential to minimize morbidity and duration of hospital stay.
The Cleaner™ device combines mechanical action with thrombolysis, largely confining the lytic drug to the local treatment area, and reducing the duration of therapy from days to minutes. Using antegrade approach, the residual lytic agent is aspirated from the local treatment area, thus preventing excess systemic exposure to the drug. Besides this, aspiration can be used to remove the remaining pieces of clot. Although effective at clearing clot, the device potentially may cause microemboli.
Even in late coming patients with subacute thrombosis like this cohort’s population, this device can be used with acceptable thrombus resolution rates. Based on the present
data, the Cleaner™ system could prove to be a safe and feasible single-session pharmacomechanical thrombolysis technique for the treatment of acute DVT in broader patient populations, and warrants further investigation in large-scale studies.


