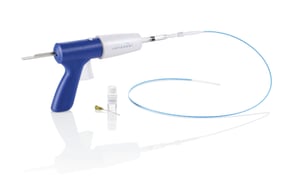
In February 2015, Covidien (now Medtronic) received pre-market approval (PMA) from the U.S. Food and Drug Administration (FDA) for the VenaSeal closure system, a new, minimally-invasive procedure that uses an advanced medical adhesive to close the diseased vein in patients with symptomatic venous reflux disease. The PMA came shortly after Medtronic’s acquisition of Covidien, which acquired the VenaSeal closure system from Sapheon, Inc. (Morrisville, North Carolina) in August 2014, and marked a significant milestone for the Medtronic’s endoVenous business.
 Medtronic, like many large medical device makers, often looks to expand its portfolio by seeking out an innovative startup technology that has the ability to efficiently drive new product development, clinical and economic evidence. The acquisition of VenaSeal offered a complementary technology that helps to both differentiate and strengthen our endoVenous business’ existing chronic venous insufficiency (CVI) portfolio. The U.S. market for CVI is approximately $280 million and is expected to grow by as much as 10 percent per year. Minimally invasive, non-tumescent, non-thermal procedures are increasingly attractive to physicians and patients, who are often able to return normal activities shortly after the procedure.
Medtronic, like many large medical device makers, often looks to expand its portfolio by seeking out an innovative startup technology that has the ability to efficiently drive new product development, clinical and economic evidence. The acquisition of VenaSeal offered a complementary technology that helps to both differentiate and strengthen our endoVenous business’ existing chronic venous insufficiency (CVI) portfolio. The U.S. market for CVI is approximately $280 million and is expected to grow by as much as 10 percent per year. Minimally invasive, non-tumescent, non-thermal procedures are increasingly attractive to physicians and patients, who are often able to return normal activities shortly after the procedure.
The VenaSeal closure system safely and effectively treats varicose veins.I, II, III, IV Unlike other treatments, VenaSeal does not require tumescent anesthesia or sedatives, allowing patients to return to work and normal activities shortly following the procedure.V, VI, VII The VenaSeal procedure also eliminates the risk of nerve or other heat-related injury associated with thermal-based procedures. Additionally, the VenaSeal may eliminate the need for compression stockings post-procedure.VIII, IX, X*
Evaluating Sapheon
Before acquiring Sapheon, Covidien assembled a due diligence team to evaluate the company. The team consisted of representatives from many functional areas, including Clinical, Regulatory, Marketing, Operations and Finance to evaluate all aspects of the company and its business, including its commercial activities, innovation and the clinical data associated with the procedure.
Medtronic Inc. Commercial Activities
At the time of the Sapheon acquisition, the VenaSeal closure system was approved for use in Australia, Canada, Europe (CE Mark, September 2011) and China (Hong Kong). In fact, more than 2,000 patients had been treated with the system at that time. We saw this as an attractive opportunity as our endoVenous business offered a strong, dedicated sales team, physician training and patient education support that could help support U.S. launch once FDA approval was obtained.
Innovation, The Medtronic Inc. Way
Investment in a non-tumescent, non-thermal and nonsclerosant procedure provides Medtronic’s endoVenous business with an unmatched product portfolio and a complementary technology to our market-leading Venefit™ procedure. The Venefit procedure uses radiofrequency ablation to close the vein without the need for vein stripping surgery, heat or harsh chemicals to close the vein. The five-year results of the ClosureFast™ Long-Term European Multi-Center Study demonstrate the durability of the procedure, with strong closure rates of the great saphenous vein and minimal treatment side effects.
Clinical Data
The VenaSeal closure system has been proven effective in three clinical studies, with demonstrated safety and high closure rates.XII, XIII, XIV, XV As a Class III device, the VenaSeal closure system required a PMA from the FDA. To support this submission, Sapheon conducted the VeClose pivotal study, a prospective, randomized study comparing the VenaSeal closure system to radiofrequency ablation. The study enrolled 242 patients at 10 sites across the U.S. The three-month results of the VeClose pivotal study published in the Journal of Vascular Surgery demonstrated the safety and efficacy of the VenaSeal procedure with closure rates of 98.9 percent.XVI
Data from the VeClose study was comparable to other clinical trials conducted on the VenaSeal closure system. These included:
- The feasibility study for the VenaSeal™ closure system— designed as a prospective, single-center study to demonstrate the feasibility, safety and efficacy — enrolled 38 patients. Results of the trial demonstratedthe safety and efficacy of the procedure, with closure rates of 92 percent at both 12 and 24 months.XVII, XVIII
- European Sapheon Closure System Observational Prospective (eSCOPE) was a prospective, multicenter, single-arm study that enrolled 70 patients at seven sites in Europe. The study demonstrated that the VenaSeal™ closure system had a cumulative closure rate of 92.9 percent and improvement in quality of life scores at 12 months.XIX
What’s Next for VenaSeal?
Since the acquisition of Sapheon, Medtronic’s endoVenous business has achieved various milestones, including:
- The publication of 12-month feasibility results in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
- The 12-month results from the eSCOPE study were also published recently in the Journal of Vascular Surgery: Venous and Lymphatic Disorders
- PMA by the FDA for the VenaSeal closure system was achieved in February 2015 ahead of schedule, and Medtronic is working towards launching the product in the U.S.
With two clinically proven technologies – the VenaSeal closure system and the Venefit procedure, Medtronic is a market leader in the endoVenous space, committed to offering treatments that deliver clinically proven results with improved patient comfort and rapid recovery.
About Cyanoacrylate Adhesives
The use of adhesives is not new in medical procedures. Cyanoacrylate adhesives have been used
successfully for many years in a variety of medical applications, including ophthalmic use, wound sealing
and tissue closure.
Characteristics of an ideal CVI adhesive include:
- High viscosity – to prevent embolization outside the treatment area and allow good contact with the intimal surface
- Quick polymerization – to prevent embolization outside the treatment area
- Soft and elastic after polymerization – to prevent the ability to feel the adhesive after implantation
- Strong bond formation – to prevent recanalization and the requirement for post-treatment compression stockings (Source: Medtronic plc)*
*Some patients may benefit from the use of compression stockings.
I Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2013;1:174-180.
II Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology / Venous Forum of the Royal Society of Medicine 2014.
III Proebstle TM, Alm J, Dimitri S et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders.
IV Gibson K. A Randomized, Controlled Study Comparing Cyanoacrylate Adhesive Embolization With RadiofrequencyAblation For Treatment Of Incompetent Great Saphenous Veins VeClose Study. German Society of Phlebology 2014.
V Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2013;1:174-180.
VI Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology / Venous Forum of the Royal Society of Medicine 2014.
VII Proebstle TM, Alm J, Dimitri S et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders.
VIII Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2013;1:174-180.
IX Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology / Venous Forum of the Royal Society of Medicine 2014.
X Proebstle TM, Alm J, Dimitri S et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders.
XI Proebstle, T. M. et al. Five-year results from the prospective European multicentre cohort study on radiofrequency segmental thermal ablation for incompetent great saphenous veins. British Journal of Surgery. February 2015. 1365 2168. http://dx.doi.org/10.1002/bjs.9679
XII Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2013;1:174-180.
XIII Proebstle TM, Alm J, Dimitri S et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders.
XIV Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology / Venous Forum of the Royal Society of Medicine, 2014.
XV Morrison, N. et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). Journal of Vascular Surgery. January 30, 2015. DOI: http://dx.doi.org/10.1016/j.jvs.2014.11.071
XVI Morrison, N. et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). Journal of Vascular Surgery. January 30, 2015. DOI: http://dx.doi.org/10.1016/j.jvs.2014.11.071
XVII Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2013;1:174-180.
XVIII Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Two-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology / Venous Forum of the Royal Society of Medicine 2014.
XIX Proebstle TM, Alm J, Dimitri S et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders.


