by Isaac K Nyameke, MD and Stephen J Goodyear, MD
Venous thrombembolism (VTE) can occur with occasional devastating consequences after any interventional procedure, including venous treatments. Therefore, VTE prophylaxis should be considered for all patients with varicose veins who are at an increased additional risk. The relative rarity of VTE events following minimally invasive endovenous procedures makes this a difficult area for study, and there is little published data on preventative measures and outcomes.[1]
The absence of trial evidence and specific published guidelines for thromboprophylaxis in patients undergoing ambulatory endovenous procedures has resulted in very variable thromboprophylaxis management in current endovenous practice. VTE guidelines from the United Kingdom National Institute of Health and Care Excellence (NICE) and the American College of Chest Physicians (ACCP) focus on in-patients undergoing major surgery rather than day case ambulatory venous interventions.[2,3]
Existing guideline advice for surgical day cases performed under local anesthesia generally recommends against giving any additional VTE prophylaxis. However, endovenous interventions are unique amongst ambulatory interventions in posing a specific VTE risk because of their disruption of superficial venous endothelium that is immediately adjacent to the deep veins.
SIGN Guidelines on Open Varicose Vein Surgery
The Scottish Intercollegiate Guideline Network (SIGN) guidance does make specific recommendations for patients undergoing open varicose veins surgery. SIGN advocates graduated compression stocking (GCS) use only, unless additional thromboembolic risk factors exist when heparin thromboprophylaxis is added.
SIGN guidance says “there is no evidence that the incidence of DVT following endovenous treatment is any different from that following open surgery,” and recommends their thromboprophylaxis strategy for endovenous interventions.[4] However evidence for this guidance is from non-analytic studies and expert opinion only.
Sparsity of direct guidance and the limited invasiveness of modern endovenous treatments (with almost immediate return to pre-operative mobility levels) have led some vein specialists to question whether any specific anti- DVT prophylaxis is ever needed for patients undergoing ambulatory endovenous treatment.
Conversely, others routinely give all patients a single dose of pharmacological prophylaxis without any attempts of clinical justification.
The Threat of VTE Complications
In reality, VTE remains a serious complication of endovenous procedures, even when performed under local anesthesia, and reports of adverse outcomes in relatively young patients appear sporadically in the popular press and in publications.[5-7]
Although rare, the potential seriousness of these outcomes attest to the need for a patient-centered thromboprophylaxis strategy in this population. The current absence of good evidence and, at best, the limited existing professional consensus should be cause for significant concern for all practicing vein specialists.
“The absence of trial evidence and specific published guidelines for thromboprophylaxis in patients undergoing ambulatory endovenous procedures has resulted in very variable thromboprophylaxis management in current endovenous practice.”
Commonly utilized strategies for managing perioperative venous thromboembolism risk in general surgical practice, such as graduated compression stockings (GCS) and anticoagulant thromboprophylaxis, have historically been adopted for open varicose vein surgery performed under general anesthesia. Some modern phlebologists have directly applied this management strategy to their local anesthetic endovenous practice.
A recent Cochrane review showed GCS significantly reduces perioperative DVT in general surgical patients, however it remains unknown whether GCS confer similar additional benefit over aggressive mobilization alone after local anesthetic endovenous treatment.[8] Low molecular weight heparins (LMWH) represent the mainstay of pharmacological anticoagulant thromboprophylaxis in general surgery and delivers significant reductions in post-operative DVT.
Caprini’s text on relative efficacy of thromboprophylaxis methods provides a good summary of the subject, and shows total DVT incidence to be significantly reduced with GCS, and more so with LMWH when compared to placebo.[9]
 The best rational strategies for employing these techniques in general surgical practice are usually best based on individual risk stratification, with higher-risk individuals receiving targeted mechanical and LMWH thromboprophylaxis. Such risk stratification is possible using risk assessment models that are based on the intended procedure or on individual patient-associated risk factors.
The best rational strategies for employing these techniques in general surgical practice are usually best based on individual risk stratification, with higher-risk individuals receiving targeted mechanical and LMWH thromboprophylaxis. Such risk stratification is possible using risk assessment models that are based on the intended procedure or on individual patient-associated risk factors.
Procedure-based assessments depend on factors such as operation site, type, and duration—with major pelvic or lower limb procedures, extended duration of surgery and prolonged post-operative immobilization signaling elevated risk. Procedure-based models are of limited usefulness for our local anesthetic endovenous interventions that are not majorly invasive.
They typically last less than 60 minutes and are usually followed by immediate mobilization. Likewise, patient-based risk assessment models such as the U.S. Caprini score and the UK Department of Health’s VTE tool are best suited for patients who are undergoing inpatient care because many of the scoring risk-factors are not applicable to patients undergoing endovenous intervention.
The Worcester VTE Risk Score
Developing risk assessment tools that focus upon factors that are more directly applicable to endovenous patients would provide specific and relevant risk stratification, and should be a priority for all practicing endovenous specialists. As a direct response to this unmet need, our current work stream has been to develop and investigate a simple scoring system (Worcester Score) produced from questionnaire surveys of British and European vein specialists, using relevant factors that were most frequently cited as being of relevance for VTE.[10]
The resulting proposed Worcester protocol is based on weighted factors with patients scoring one point for each of the following factors: obesity (defined as a BMI of >30), oral contraceptive or hormone replacement therapies known to increase VTE risk, and active (symptomatic) superficial vein thrombosis (thrombophlebitis).
Patients score two points for each of the following factors: history of proven VTE, compromised calf muscle pump function (defined as an inability to walk normally to and from the intervention room), and known thrombophilia. Patients’ total scores are then used to simply stratify their risk as low (total score: 0), moderate (total score: 1) or high (total score: >2).
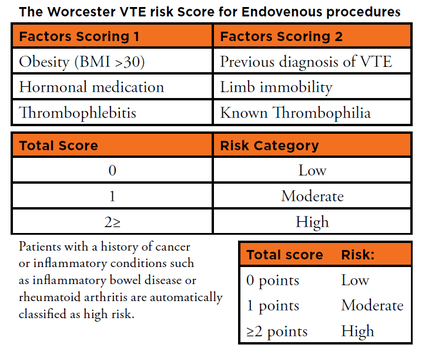
Patients with a history of cancer, or any underlying inflammatory condition such as inflammatory bowel disease or rheumatoid arthritis, are automatically assigned high risk status.
Our management strategy is that after endovenous intervention, all able patients should be immediately mobilized and suitable patients should wear appropriate GCS unless there is a specific contraindication such as significant peripheral vascular disease.
We advocate administration of additional LMWH thromboprophylaxis for patients who have been scored as moderate or high risk status.
“In reality, VTE remains a serious complication of endovenous procedures, even when performed under local anesthesia, and reports of adverse outcomes in relatively young patients appear sporadically in the popular press and in publications.”
The duration of prescribed LMWH thromboprophylaxis is another area of controversy and variable practice following endovenous treatment. Once the decision has been made to give additional LMWH, what should be the duration of treatment?
In a 2014 vote among vein specialists of the UK Venous Forum, a clear majority of practitioners favored single-dose LMWH for VTE thromboprophylaxis (whether this was being given selectively or universally), despite conflicting evidence from the hematology literature.
Evidence, such as epidemiological data on the duration and magnitude of post-operative VTE risk in middle aged women, shows that VTE risk is substantially increased and for a prolonged period after the index intervention.
Indeed, Sweetland et al in the Million Women Study showed that, compared to non-operated patients, this increase is greater than seven-fold after day case surgery and is prolonged for several weeks into the post-operative period.[11] The pharmacokinetics of LMWHs are that their plasma antifactor Xa activity generally ranges from four to six hours after subcutaneous injection.[12]
This time is substantially less than the duration of the patient’s VTE risk. This renders the practice of administering single-dose LMWH to patients who are assessed as being at an increased risk for VTE after endovenous treatment largely ineffective during most of their extended high-risk period.
This data provides the basis for us to argue that prescriptions for LMWH (for selected patients) be extended beyond the “single shot,” and into the postoperative period. The optimum duration of this extension, following ambulatory endovenous intervention, has yet to be determined, however our current practice is to extend treatment for one week for moderate risk patients and up to four weeks for high risk patients.
Conclusion
 In summary, ambulatory endovenous treatments have the potential to induce VTE, with occasional devastating sequelae. All patients scheduled for endovenous intervention should be assessed to determine their VTE risk, and patients who are identified as being at an increased risk should be given appropriate thromboprophylaxis risk management.
In summary, ambulatory endovenous treatments have the potential to induce VTE, with occasional devastating sequelae. All patients scheduled for endovenous intervention should be assessed to determine their VTE risk, and patients who are identified as being at an increased risk should be given appropriate thromboprophylaxis risk management.
However, sparseness of applicable evidence and guidance and limitations in the relevance of current risk-stratification models have led to variable practice and controversy that may potentially negatively impact VTE outcomes after endovenous treatment.
There is an urgent need for tailored risk assessment models for this patient group, such as the proposed simple ”Worcester Score” described earlier, with a view to standardizing rational and safe thromboprophylaxis practice, and as a starting point to generate evidence for managing this important potential complication.
Once patients have been categorized for VTE risk, we advocate additional LMWH thromboprophylaxis for all moderate risk and high risk patients. This LMWH treatment needs to be continued into the post-procedure period because the at-risk period is prolonged well beyond the duration of action of single-dose LMWH.
References:
- Sutton PA, El-Dhuwaib Y, Dyer J, Guy AJ. The incidence of post-operative venous thromboembolism in patients undergoing varicose vein surgery recorded in Hospital Episode Statistics. Ann R Coll Surg Eng 2012; 9 4(7): 481–83.
- National Institute for Health and Care Excellence. Venous thromboembolism in adults: reducing their risk in hospital (Quality Standard 3). http://bit.ly/2kNctd2 (date accessed 08.02.17)
- Arcelus JI, Heit JA, Samama CM; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 141(2 Suppl):e227S-77S.
- Scottish Intercollegiate Guideline Network. Guideline 122: Prevention and management of venous thromboembolism: http://www.sign.ac.uk/pdf/sign122.pdf (date accessed 08.02.17).
- The Daily Mail. Mother-of-two’s death after varicose vein surgery ‘could have been prevented,’ her husband claims. http://www.dailymail.co.uk/health/article-2980924/Mother-two-s-death-varicosevein-surgery-prevented-husband-claims.html (date accessed 08.02.17).
- RTE News. Inquest into the death of Karen McCabe adjourned until May. http://www.rte.ie/news/2016/0218/769058-inquest-varicose-veins-surgery-death (date accessed 08.02.17).
- Spinedi L, Staub D, Uthoff H. Successful lysis in a stroke following endovenous laser ablation and extensive miniphlebectomy of varicose veins. Phlebology. 2016 May;31(4):296-8
- Sachdeva A, Dalton M, Amaragiri SV, Lees T. Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No.: CD001484. DOI: 10.1002/14651858.CD001484.pub3.
- Caprini JJ. Chapter 38: Thrombotic Risk Assessment: A Hybrid Approach. The Vein Book (ed. John J Bergan and Nisha Bunke- Paquette). Pp 295; 2014 Oxford University Press
- Goodyear SJ. I Nyamekye, (2016) Rational anti-DVT prophylaxis for ambulatory varicose veins procedures. In: Greenhalge R. M. (Ed.)Vascular and Endovascular Challenges Update. London: BIBA Publishing, pp. 541-549
- Sweetland S, Green J, Liu B, Berrington de González A, Canonico M, Reeves G, Beral V; Million Women Study collaborators.Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009 Dec 3;339: b4583.
- Weitz JI. Low-molecular-weight heparins. N Engl J Med 1997;337:688–698.




