Over the past 25 years arterial stent introduction systems have changed considerably. From the first 7F for 10mm diameter Palmaz and Wallstents, we have now shrunk down to 10mm through 4F on 0.018 wires. This has occurred because the wire lumen has decreased in size from 0.035” to 0.018”, stent struts have become thinner, and engineering specifications have grown tighter.
Radio-opacity has decreased, with tantalum/titanium markers now guiding us as to the ends of the stents. This has been possible because it was realized that arterial stent placement was acting as a scaffold to stabilize the deliberate cracking of arterial wall plaque. Recoil forces are low. Stenting confers slightly better results than angioplasty in the iliac arterial system, mainly by decreasing the rate of angioplasty-induced dissection.
It couldn’t be much more different in the venous world. Up until four years ago, in Europe, the Wallstent was still the number one venous stent—in the United States, it has almost always been number one. But then, in fairly quick succession, “dedicated” venous stents obtained CE marking (European equivalent of the FDA). These were, in order of launch: Cook Medical Zilver Vena (Limerick, Ireland), OptiMed Sinus Venous (Karlsuhe, DE), and the Veniti Vici (St. Louis, Missouri). Boston Scientific obtained a retrospective CE for venous indication on the Wallstent in 2015.
Dedicated Venous Stents
So are the new “dedicated” venous stents really that different? Well, yes, they are.
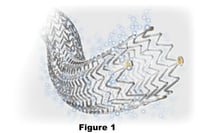
The Cook Zilver Vena is an open-cell, laser-cut nitinol, self-expanding stent on a 6F/0.035” platform (see figure 1). It is very precise, shows zero foreshortening, has 30% higher radial force than the Cook Zilver, and the results of the VIVO EU study have been encouraging. It has excellent wall conformability and trackability (see figures 2, 3). It is available in 14 and 16mm diameters and 60, 100 and 140mm lengths. This stent is currently on a multicentre U.S. FDA trial and enrollment is at about 75%, so results should be available in the next 18 months or so.
The OptiMed Sinus Venous is a different concept. It comes on a 10F platform and consists of two open–cell, laser-cut nitinol stents with high radial force linked in just one spot to the next set of two stents (see figure 4). The connection between the two is called a “flash link,” which adds flexibility to the system. It is available from 10-18mm diameter and ranges from 60-150mm long with zero foreshortening.
Results from this stent system have been published recently by de Graaf et al. This paper concerned ilio-caval reconstruction, in which the Maastricht group specializes. They achieved 100% patency at 6/12, which is seriously impressive. In addition, they cleverly added a balloon-expandable (Andramed) section to the kissing portion to prevent one self-expanding stent from compressing the other.
OptiMed has also produced a further array of venous stents, including the Sinus XL (closed-cell, large diameters; high radial force, only for straight vessels-aorta/IVC), or Sinus XL Flex (can accommodate curves). The most recent iteration is the Sinus Obliquus, which is specifically designed for left-sided May-Thurner lesions. This stent is composed of an obliquely-cut Sinus XL married to a standard Sinus Venous (see figures 5, 6). This design should lessen the approximately 1% incidence of contra-lateral right common iliac vein (RCIV) thrombosis by covering less of the orifice of the RCIV.
Veniti is a venous-focused company from St. Louis, MO. They manufacture a 10F, 0.035” platform, laser-cut, open-cell nitinol, self-expanding stent (see figures 4-6). This stent does show some foreshortening (approximately 10-15%), which is considerably less than the Wallstent (30-40%). The stent demonstrates considerable outward radial force (see figures 7-10). The European and US trials are well underway, and FDA approval should follow in due course.
Other companies interested in the IVF arena
Bard has already achieved CE marking with their “Venovo” stent (Q3/2015), and a clinical trial (Vernacular) is about to start in Europe. This is a laser-cut nitinol, open-cell design with significant radial force, but good conformability. In keeping with other manufacturers, they have produced 12-16 mm diameters with a considerable array of lengths. I do not have clinical experience with this stent. In vivo-arriving in Galway next week apparently- deployment is through a ratchet-type roller wheel with single-handed deployment, which is different from the rest.
Medtronic has produced—what is by now familiar to you as the standard—self-expanding, laser-cut nitinol stents with a standard array of lengths and diameters. This should be released in Q1/2 2016. It appears to have good conformability and radial hoop strength ex vivo.
There are rumors that both Cordis and Gore have “venous stents” in development. And, again, rumors abound that BSCI may partner the long-established Wallstent with a newer laser-cut nitinol, venous-type stent.
So one can see a common direction, but we are so early that who knows where things will be in 3-5 years. The rapid proliferation of so many devices clearly means the companies believe that venous stent placement is going to grow. Obviously the ATTRACT trial will increase the visibility of venous disease, and recent trials and experience (BERNUTIFUL trial) and work reported by Michael Lichtenberg (Arnsberg, DE) shows that the rate of venous stenting post-EKOS ultrasound-assisted thrombolysis and Straub-Aspirex venous thrombectomy is over 90%.
What this means is that if more patients have venous thrombectomy/lysis, more patients will get stents. And there is a massive untapped market of patients with previous ilio-femoral venous occlusions who will become more aware that they have potentially curative options in place of simple anti-coagulation and compression stockings.
In practical terms, use of the newer stents has certainly grown in Europe, but the Wallstent remains a favorite not only because it is the most well-established stent with easily the longest published series, but also because of pricing, which remains quite high for the new stents.
Future directions for IVF
We are at a stage equivalent to 1991 in coronary artery stents. Think of all that has occurred since then! We have no idea as to the proper place of covered stents, drug-eluting stents, branched stents, bifurcation stents, or precise stent sizing. Nor do we even have a solid basis for anticoagulation vs. anti-platelet therapy pre- and post-stent placement. All that we are certain of is that nothing is certain!



