 Reports of chronic venous disorders (CVD) date to biblical times, but beginning with the era of industrialization, CVD has developed into one of the most widespread causes of human disability and discomfort. The breadth of CVD (including lymphatic complications) encompasses spider, reticular and varicose veins, trophic skin changes, leg ulcers, pitting edema, leg pain, joint pain, congestion, skin irritation, and itching, as well as restless legs and muscle cramps.1 Of greater concern, venous stasis can lead to deep vein thrombosis and a greatly increased risk of pulmonary embolism. Epidemiologic studies have shown that more than half of adults in the western world have CVD associated symptoms that are severe enough to reduce their quality of life, with women and the elderly significantly more affected than men.2 Correspondingly, CVD result in substantial medical and economic challenges, with venous insufficiency accounting for more than $3 billion alone in annual direct costs in the U.S.3
Reports of chronic venous disorders (CVD) date to biblical times, but beginning with the era of industrialization, CVD has developed into one of the most widespread causes of human disability and discomfort. The breadth of CVD (including lymphatic complications) encompasses spider, reticular and varicose veins, trophic skin changes, leg ulcers, pitting edema, leg pain, joint pain, congestion, skin irritation, and itching, as well as restless legs and muscle cramps.1 Of greater concern, venous stasis can lead to deep vein thrombosis and a greatly increased risk of pulmonary embolism. Epidemiologic studies have shown that more than half of adults in the western world have CVD associated symptoms that are severe enough to reduce their quality of life, with women and the elderly significantly more affected than men.2 Correspondingly, CVD result in substantial medical and economic challenges, with venous insufficiency accounting for more than $3 billion alone in annual direct costs in the U.S.3
In addition to age and gender, both the duration of daily sitting and standing along with lack of exercise are consistent independent predictors of CVD.4 Postural orthostasis plays a critical role in the development of venous disorders because a principle underlying factor in essentially all venous disorder related conditions is increased venous pressure.5 Despite this, there have been remarkably few interventions developed to prevent venous hypertension. Leg elevation, of course, reduces venous pressure but is generally not viewed as a practical approach for individuals in either the work or home environment. As a result, compression therapy was introduced as a means to reduce systolic pressure peaks and decrease extravasation into the interstitial space. It has, over time, become the gold standard for non-invasive treatment of CVD. Compression interventions include not only stockings, but also multilayer bandage systems, paste bandage systems and pneumatic compressive devices. Compliance with compression stockings and bandaging is typically very poor. While pneumatic compression has historically involved the use of a bulky apparatus, which limited mobility and therefore compliance, new portable technologies are currently under development.6
 Despite widespread utilization of compression interventions, neither invasive nor non-invasive studies have been able to demonstrate any significant changes in ambulatory venous pressures or venous recovery times resulting from compression therapy.7 This raises the question of the underlying physiologic basis of venous hypertension and what might be the most effective intervention strategies to preclude the numerous health complications associated with CVD.
Despite widespread utilization of compression interventions, neither invasive nor non-invasive studies have been able to demonstrate any significant changes in ambulatory venous pressures or venous recovery times resulting from compression therapy.7 This raises the question of the underlying physiologic basis of venous hypertension and what might be the most effective intervention strategies to preclude the numerous health complications associated with CVD.
Venous hypertension is a hydrostatic phenomenon. Three physical factors, relatively unique to humans, influence the development of venous hypertension.8 First, is the location of our heart, which, in upright posture, is sufficiently high in the body with more than 70 percent of the blood in the cardiovascular system remaining below the heart. The large size of our legs (about half our body volume), and the high compliance of human veins, allows most of this blood to collect into the veins of the legs.
Second is the exceptional height of humans, which results in venous columns of blood over one meter in height (distance from feet to right atrium) in even the shortest individuals. Gravity, operating on these fluid columns during orthostasis creates static venous pressures of 100 mmHg or more in the feet and lower legs.
Finally, human skin is extremely compliant; if human skin were stiff (had low compliance) like that in other tall animals (horses, cattle, giraffes, ostrich, etc), high static venous pressures would present minimal difficulties. However, the combination of these three factors means that during orthostasis, the large hydrostatic pressures in the veins and capillaries drive venous and capillary expansion, leading to increased extravasation, and our compliant skin, particular that of women, stretches out, accommodating extensive blood and interstitial fluid pooling to the point where venous return to the heart and cardiac output are compromised.
Further exacerbating this process, both our veins and skin have visco-elastic properties and thus undergo stress-relaxation (often referred to as creep) when exposed to static pressures for extended time periods, leading to additional fluid pooling during extended orthostasis. The rate at which the immediate hydrostatic effects develop is dependent on a number of physiologic and environmental factors. For the initial pooling phase when blood flow is normal, the full hydrostatic effects can take minutes for the veins to fill past the point where the venous valves no longer function; in hot environments, the veins can fill to the point where venous valves become incompetent within several seconds. The slower, stress-relaxation processes occur over a timescale of hours.
Given this hydrostatic basis of venous hypertension, it is clear that compression therapy can serve to reduce effective skin compliance, thereby allowing interstitial pressures to increase, leading to a self-limiting of lower limb blood pooling. However, high interstitial fluid pressures reduce the vascular transmural pressure gradients and physically compress capillaries, resulting in reduced nutritive tissue perfusion.9 Intermittent pneumatic compression can help prevent this loss of nutritive tissue perfusion, but none of the compression approaches address the fundamental physiologic failure leading to the development of venous hypertension.
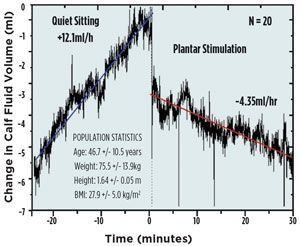
Figure 1. Effect of plantar micromechanical stimulation
on lower limb fluid pooling in adult women. Initiation of
stimulation (HeartPartner, Sonostics, Inc) at time 0 leads
to a rapid drop in calf fluid volume as a result of enhanced
venous return, followed by a slower, sustained decline
associated with enhanced lymphatic return (from Ref. 13).
In healthy individuals, under normal physiologic conditions, pooling of blood and interstitial fluid into the lower limb veins during orthostasis is limited by the action of the calf muscle pumps (soleus muscles). These deep postural muscles serve to continually return blood and interstitial fluid back to the heart against the large hydrostatic pressure gradient which exists when in upright posture. While reflex voluntary muscle contraction occurs when one first sits or stands up, creating muscle tone which can transiently limit venous pooling, these muscles fatigue over a few tens of minutes, leaving the soleus muscles as the primary muscle pump in the lower leg. In fact, the soleus muscles alone are responsible for more than 70 percent of the fluid return from the lower body during orthostasis, with the foot and thigh pumps contributing the remainder. These muscles play such a critical role in the return of venous and lymphatic fluid to the heart that they are often referred to as our “secondary hearts.”
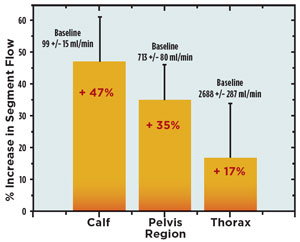
Figure 2. Enhanced fluid return from the legs reduces lower
limb tissue pressures and increases cardiac output, resulting
in significantly increased tissue perfusion in the lower body,
as represented by impeding plethysmographic assessments
of lower limb, pelvic and thoracic blood flow. (from Ref. 14).
A primary reason why our modern, western European lifestyle has led to such a large increase in CVD is that we now rarely utilize our soleus muscles in daily life. While our ancestors commonly squatted while working or resting, western Europeans developed the habit of sitting in chairs.10 The average American adult now sits for more than 13 hours a day. The sitting position precludes gastrocnemious contraction due to the insertion of this muscle on the femur, and thus prevents the gastroc from contributing to any venous/lymphatic pumping. Similarly, sitting does not involve any extensive soleus muscle activity. Conversely, squatting requires significant soleus muscle activity, and in fact, the soleus becomes the dominating lower limb muscle during squatting activities.11
Similar to the cardiac muscle, the soleus muscles are highly specialized, containing large venous sinuses which fill with blood and lymphatic fluid, then in response to a stretch reflex, contract. Unlike the cardiac muscle, the cyclic contraction rate of the soleus muscle is very slow, with the filling times and contraction times occurring over minutes. As a result, most people are unaware of this cyclic soleus activity and correspondingly, any decline in soleus activity. The first indications of soleus failure include the development of lower limb swelling due to interstitial fluid buildup, varicose veins due to venous blood pooling, fatigue and other complications associated with reduced cardiac output, or cognitive disorders associated with the chronic hypotension arising from low cardiac output.
The origin of CVD, therefore, most commonly arises through the loss of normal soleus muscle function. But, as with any other skeletal muscle, the soleus muscles can be retrained. These are deep postural muscles, and so the required training regimen involves only low level, but long duration, exercise. The most efficacious training exercises, not surprisingly, are squat exercises. Squatting exercises can be difficult for many people, so a closely related alternative is Tai Chi which has been shown to significantly enhance soleus performance in older adults.12 If a more passive approach is desired, direct, transcutaneous electrical stimulation (EMS) of the soleus muscles can be utilized. Soleus insufficiency is typically reflected in a transition from slow twitch (1A) fibers being dominant in the muscle to fast twitch (11B) fibers dominating. Sustained EMS of the soleus muscles has been shown to result in reconversion to type 1A fibers, as well as increasing glycogen supplies in the muscle.13 EMS for muscle re-education has been approved by the FDA and available to clients on a prescription basis.
Despite the demonstrated efficacy of EMS, its effectiveness is limited as a result of many individuals caring for the sensation of having their muscles externally stimulated. In addition, external stimulation, by not utilizing physiologic muscle control pathways, results in rapid muscle fatigue. While the latter may not be an issue when retraining voluntary muscles, it is a significant issue when attempting to retrain a deep postural muscle which requires several hours per day of activation.
An alternative approach to retraining the soleus is to rely on a physiologic control pathway. While the stretch reflex is typically non-functional in individuals with soleus insufficiency, the postural reflex is invariably intact as evidenced by the fact that most individuals with soleus related symptoms can maintain a standing posture. The soleus postural reflex arc begins on the sole of the feet where mechanoreceptors, specifically Meissner’s Corpuscles, are activated by foot pressure. Pressure on the forefoot leads to a firing of the soleus muscle, while pressure on the heel leads to a relaxation of the soleus muscle. This reflex can be activated by micromechanical stimulation of the Meissner’s Corpuscles on the frontal plantar surface while an individuals is sitting, leading to soleus contraction through both short and long loop reflex arcs. The reliance on the reflex arcs prevents fiber fatigue during prolonged stimulation. Therefore, this approach offers a convenient means to provide the two hours or more each day required to re-educate the soleus muscles. It is important for the individual to continue to wear footwear as the micromechanical stimulation readily passes through socks, slippers and the soles of shoes.
The ability of micromechanical reflex stimulation to activate the soleus muscles is evident in studies of reduction of edema and enhanced circulatory flow during stimulation. Using air plethysmography to track changes in calf volume, the fluid pooling in the lower leg is not only stopped, but reversed by soleus muscle stimulation (Figure 1).13 The removal of fluid, both venous and interstitial, reduces the tissue pressure on the capillaries and allows enhanced circulation to not only the lower leg, but throughout the lower body as shown through electrical impedance plethysmography studies (Figure 3).14 Moreover, the stimulation can be applied in a paced manner, so that the muscle is allowed time to refill with venous blood and lymphatic fluid before contraction is initiated. This technology is currently available as an over-the-counter intervention for prevention of CVD, for example, Sonostics’ HeartPartner product (Figure 3).
In summary, the impact of CVD continues to grow in the modern, industrial world. A major factor in this development is the sedentary lifestyles associated with modern life, in particular the extended sitting times associated with working, eating, entertainment and travel. A convenient means to slow or reverse the development of venous hypertension would have a significant impact on the quality of life for tremendous numbers of people, especially given that CVD is an age-related condition, and we are facing a rapidly growing number of elderly from the baby boom generation. While compression therapies have a role to play in this challenge, recent work has demonstrated that soleus muscle dysfunction often plays a major role in the progression of lower limb fluid pooling and venous hypertension. Soleus muscles can be retrained utilizing EMS approaches, but a safer, less expensive and more convenient approach for many is likely to be micromechanical plantar stimulation.
References
- Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J Vasc Surg. 2004;40:1248- 1252.
- Rabe R (2015) The prevalence of lower-limb venous symptoms in recent epidemiological surveys. Medicographia 37:16-19.
- McGuckin M, Wateman R, Brooks J, et al. (2001) Validation of venous ulcer guidelines in the United States and United Kingdom. Am J Surg 183:132-137.
- Carpentier FH, Maricq HR, Biro C, Poncot-Makinen CO & Franco A. (2004) Prevalence, risk factors and clinical patterns of chronic venous disorders of lower limbs. J Vasc Surg 40:650-659.
- Cesarone MR, Belcaro G, Nicolaides AN, et al. (2002) ‘Real’ epidemiology of varicose veins and chronic venous diseases. Angiology 53:119-130.
- Lurie F, Schwartz M. (2017) Patient-centered outcomes of a dual action pneumatic compression device in comparison to compression stocking for patients with chronic venous disease. J Vasc Surg and Lym Dis 5:699-706.
- Nicoloff AD, Moneta GL, Porter JM (2002) Compression treatment of chronic venous insufficiency, in Handbook of Venous Disorders, 2nd Edition, P Gloviczki and JST Yao, ets. Arnold Press, NY, pp. 302-308.
- Rowell, LB (1993) Human Cardiovascular Control. Oxford University Press, NY.
- Scallan J, Huxley VH, Korthuis RJ (2010) Capillary fluid exchange. Morgan & Claypool Life Sciences, Chapter 4.
- Hewes, GW (1953) World distribution of certain postural habits. Annual Meeting of the American Anthropological Association, December.
- De Oliveira Sousa C, de almeida Ferreira JJ, Catarina A, Medeiros, LV (2007) Electromyographic activity in squatting at 40o, 60o and 90o knee flexion positions. Rev Bras Med Esporte 13:280-286.
- Chen YS, Zhou S, Cartwright, C (2011) Effect of 12 weeks of Tai Chi training on soleus Hoffman reflex and control of static posture in older adults. Arch Phys Med Rehabil 1.92:886-89.
- Goddard AA, Pierce CS, McLeod KJ (2008) Reversal of lower limb edema by calf muscle pump stimulation. J Cardiopulmonary Rehab and Prevention 28:174-179.
- Stewart JM, Karman C, Montgomery LD, McLeod, KJ (2004) Plantar vibration improves leg fluid flow in perimenopausal women. Am J Physiol Regul Integr Comp Physiol 288: R623-R62


