Gray areas in vein disease: In this series of articles, we discuss the uncertain and the unsure. Eight thoughtful, knowledgeable, and confident vein specialists contemplate four venous disease areas: superficial disease; deep disease, reflux; deep disease, post-thrombotic; and acute DVT.
DESPITE THE LACK of benefit from pharmacomechanical thrombolysis in proximal acute lower extremity DVT as reported by the ATTRACT trial, the field is more active than ever.1 Criticisms of trial design and sub-group analysis, as well as incorporation of prior clinical experience and publications have led to a consensus that can be summarized in the following points:
- Thrombus removal in acute DVT affecting the common iliac vein (CIV), external iliac vein (EIV) and common femoral vein (CFV) is worthwhile, with prompt symptomatic improvement and expected reduction in the incidence and severity of post thrombotic syndrome.
- Identification of residual thrombus and underlying anatomical factors causing venous obstruction via intravascular ultrasound is mandatory.
- Correction of the above by venous stenting reduces the risk of residual hemodynamic disturbances that can lead to re-thrombosis or create symptoms in their own
The surgical triad of optimizing inflow, conduit and outflow applies to venous interventions for DVT. Inferior vena cava (IVC) outflow is seldom a problem in first time events. Current technology can reliably restore patency and diameters to the iliofemoral venous segment. Inflow reconstruction remains as the main challenge and limiting factor to a successful intervention.
Endovenous thrombus removal from the FV and popliteal vein (PV) in continuity with the CFV and iliac veins is the approach most commonly taken.
This strategy is optimal when there is venous patency distal to the point of access, and the deep femoral vein (DFV) is uninvolved. Thrombosis distal to the point of access can be addressed by a two-level intervention using a secondary tibial vein access.
DFV thrombosis is frequently associated with iliofemoral DVT. It has been reported as present in up to 88% of such thrombotic events.2 I would like to make the case for thrombus removal from the DFV and describe a technique by which this can be easily achieved.
In the arterial realm, the deep femoral artery is considered to be the main outflow vessel to the lower extremity, given that it perfuses the more extensive muscle mass of the thigh. It is systematically favored in iliofemoral reconstructions over the superficial femoral artery, which perfuses the much smaller infrageniculate territory. Even though venous anatomy parallels the arterial, the DFV does not garner such attention. It is true that the parallel is not perfect. Flow volume through the DFV is approximately 60% of that estimated for the FV.3 That being said, the DFV contribution to CFV inflow is by no means negligible.
Due to its extensive branching pattern, the DFV can become an effective collateral in the setting of chronic FV occlusion. There is often a PV to DFV communication that allows antegrade flow and even axial transformation of the DFV, effectively reverting to embryonic anatomy.4 The role of the DFV is highlighted by the fact that when patent, in the context of optimized iliofemoral outflow, occlusion of the FV has limited consequences.5 Its preservation is therefore desirable.
Occlusion of the DFV is a known negative prognostic factor for the patency of iliofemoral venous reconstructions. Compromising the DFV ostia by stent coverage is to be avoided during iliofemoral stenting. Occlusion of the DFV, or post thrombotic changes extending to or across its origin, constitutes an indication for open endophlebectomy.6,7
In spite of its importance, the DFV is often neglected. From a diagnostic perspective, it is not commonly imaged with ultrasound beyond its most proximal portion. At the time of venography, its patency is usually inferred from reflux into it or by the presence of contrast washout. Seldom is it selectively catheterized. During open surgical thrombectomy the presence of valves and difficulty exposing distal branches for through-and-through access limit embolectomy to the cephalad portion as well.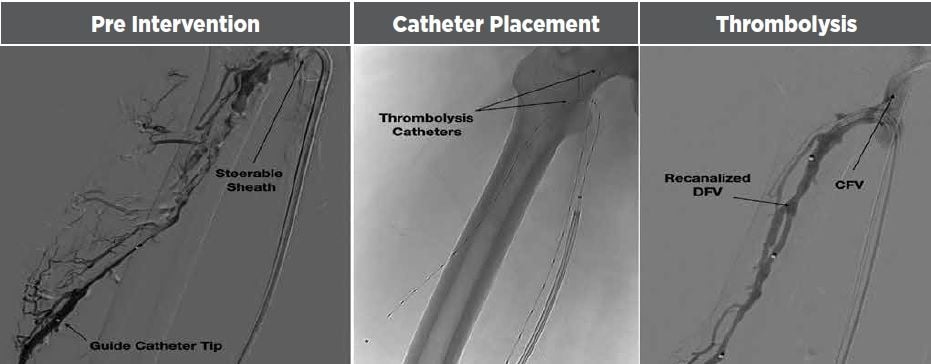
Endovascular access to the DFV can also be challenging. The width and malleability of the IVC bifurcation during up-and-over contralateral access induces poor pushability. In addition, prone contralateral popliteal access can result in issues with catheter length. Supine FV access compromises the distal extent of ipsilateral thrombus removal. Combining the two approaches generates inconveniences in patient positioning. Ipsilateral DFV wire access from either the FV or PV is unstable and generally does not allow traversing the first or second valve.
Beyond issues with establishing wire access, delivery of thrombectomy systems into the DFV is difficult due to valves and multiple branching points, limiting intervention to the initial five centimeters of vein or less.
In aggregate, these difficulties limited my past treatment of the DFV to placing an ultrasound accelerated thrombolysis catheter across the CFV in hopes there would be clearance of DFV thrombus by a proximity effect.
More recently I have been able to quickly gain catheter access to the distal DFV with the use of a steerable sheath. This device allows robust and stable engagement from an ipsilateral popliteal approach and generates enough pushability to traverse thrombus and valves to deliver angiographic or thrombolysis catheters.
With the patient in prone position, the popliteal vein is accessed and a 6.5Frx55cm steerable sheath with a 9mm deflection length is delivered to the mid common femoral vein. The tip is then angulated to mimic the DFV takeoff. Using a 90cm support catheter and a stiff, angled tip glidewire, the DFV is engaged and crossed onto its distal portion using serial venography to ensure permanence in the main trunk of the vein up to a point distal to the thrombus burden.
Following this, an ultrasound-accelerated thrombolysis catheter is placed across the thrombosed segment of DFV onto the FV. Once the catheter is in its final position, the sheath can be straightened and partially withdrawn (but not removed) to expose the full treatment length of the catheter. Generally, attempting to advance the thrombolysis catheter without steerable sheath support results in loss of wire access.
Following this stage, another popliteal vein access is stablished, and a second thrombolysis catheter is placed across the iliofemoral segment to address the more proximal thrombus burden.
Thrombolysis then takes place, splitting the tPA dose between the two catheters with a planned return to the angiographic suite after approximately 24 hours. At this time, the steerable sheath is readvanced and angled as it was for the original DFV access. Angiography is performed, and adjunctive maneuvers, mostly limited to balloon angioplasty, are carried out as necessary. After this is completed, the sheath is once again withdrawn to the FV and used for contrast injection during completion of the iliofemoral reconstruction, which is carried out through the second popliteal access.
Although still early in this experience, technical success has been gratifying. It stands to reason that regaining deep femoral vein patency should enhance iliofemoral flow, thus reducing the likelihood of iliofemoral re-thrombosis, especially in cases in which flow across the popliteal and femoral veins is suboptimal or altogether lost.
References:
- Vedantham S, Goldhaber JA, Julian SR, et al. “Pharmacomechanical catheter-directed thrombolysis for deep-vein ”New Engl J Med 2017; 377:2240-52.
- Broholm R, Baekgaard N, Hansen S, et “Significance of partial or com- plete thrombosis of the common and deep femoral vein in patients with deep vein thrombosis.” Eur J Vasc Endovasc Surg 2019; 58(4):570-575.
- Ogawa T, Lurie F, Kistner R, et “Reproducibility of ultrasound scan in the assessment of volume flow in the veins of the lower extremities.”J Vasc Surg 2002; 35:527-31.
- Raju S, Fountain TM, Neglen P, et “Axial transformation of the profunda femoris vein.” J Vasc Surg 1998; 27:651-9.
- Raju S, Ward, M, Davis, “Relative importance of iliac vein obstruction in patients with post-thrombotic femoral vein occlusion.” J Vasc Surg: Venous and Lym Dis 2015; 3:161-7.
- Vogel D, Comertota A, Al-Jabouri M, et “Common femoral endovenectomy with iliocaval endoluminal recanalization improves symptoms and quality of life in patients with postthrombotic iliofemoral obstruction.”J Vasc Surg 2012; 55:129-35.
- Dumantepe M, Aydin S, Okten M, et al. “Endophlebectomy of the common femoral vein and endovascular iliac vein recanalization for chronic iliofemoral venous ” J Vasc Surg: Venous and Lym Dis 2020; 8:572-82.


