BEFORE DISCUSSING how to obtain the best results with sclerotherapy, it is necessary to examine what the target of our treatment is. For the purpose of this discussion, we will examine telangiectasias and how to successfully treat them with minimal adverse sequalae. A short discussion of the histology and pathophysiology of telangiectasia is warranted. It is difficult to obtain good results with sclerotherapy if these basics are not understood.
A spider vein is a dilated dermal vein (Fig. 1). The characteristic histological findings are smooth muscle wall hypertrophy and a dilated lumen. The vessel wall is at least four times the normal thickness of a dermal vein. Red telangiectasis are found at a depth of 300 microns, while bluish telangiectasis are found at the mid dermal layer at around 1000 microns.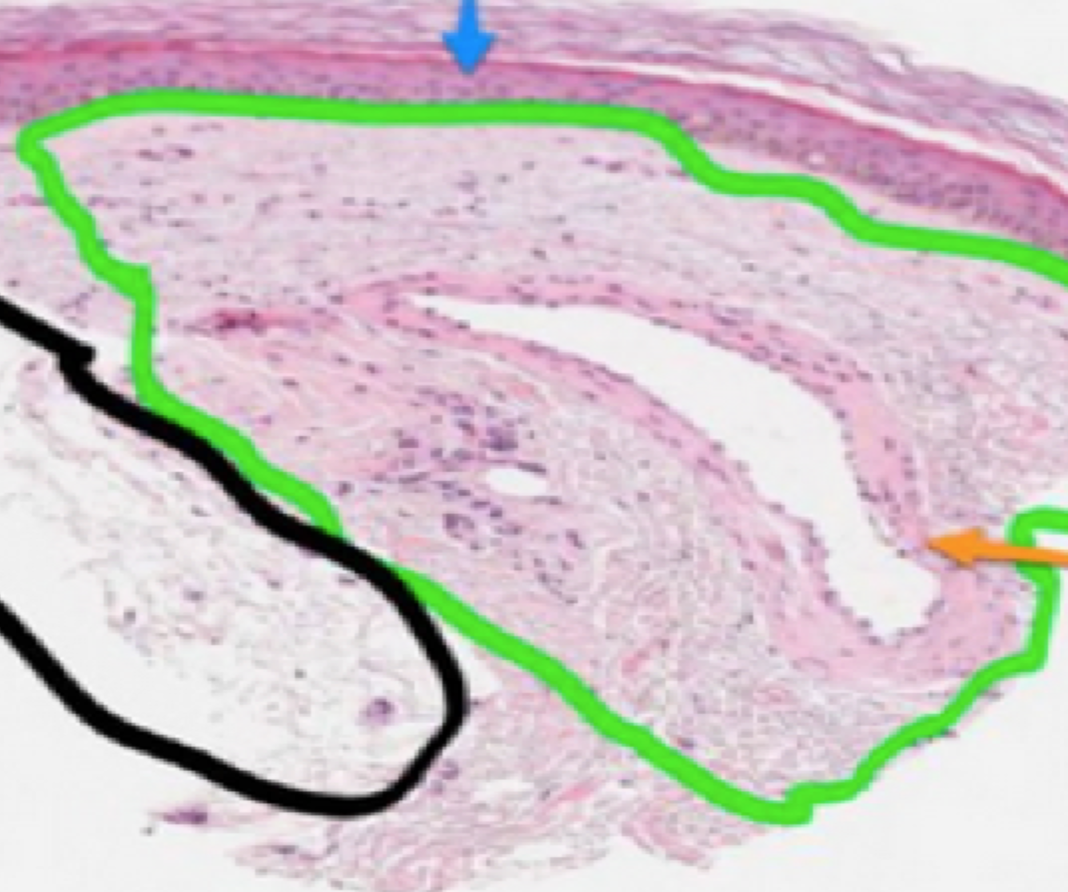
FIGURE 1 Dilated one millimeter spider vein. The green circle represents reticular dermis. The black circle represents the subdermal tissue. The orange arrow points to the spider vein. The blue arrow points to the squamous epithelium.
Telangiectasias occur due to increased pressure from a dermal perforator. These perforators dilate due to reflux and/or turbulent flow in the subdermal reticular venous network. The reticular veins may or may not be connected to a deeper source of venous reflux.
The dermal perforator is usually about one millimeter in diameter and drains a consistent anatomical area. The reason that spider veins are grouped together and fan out is that these dilated spider veins are associated with one dermal perforator that has become abnormal. Most telangiectasias are of similar length, and this phenomenon represents the equalization of pressure between the inflow (dermal perforator) and the vast cutaneous venous network. A dermal perforator is identified on duplex ultrasound (US) as a gap in the dermal tissue (Fig. 2). The dermal perforator is the etiology of all forms of cutaneous venous hypertension (CVH). CVH is the dermal manifestation of venous disease and involves the entire spectrum of venous pathophysiology from spider veins to venous ulceration.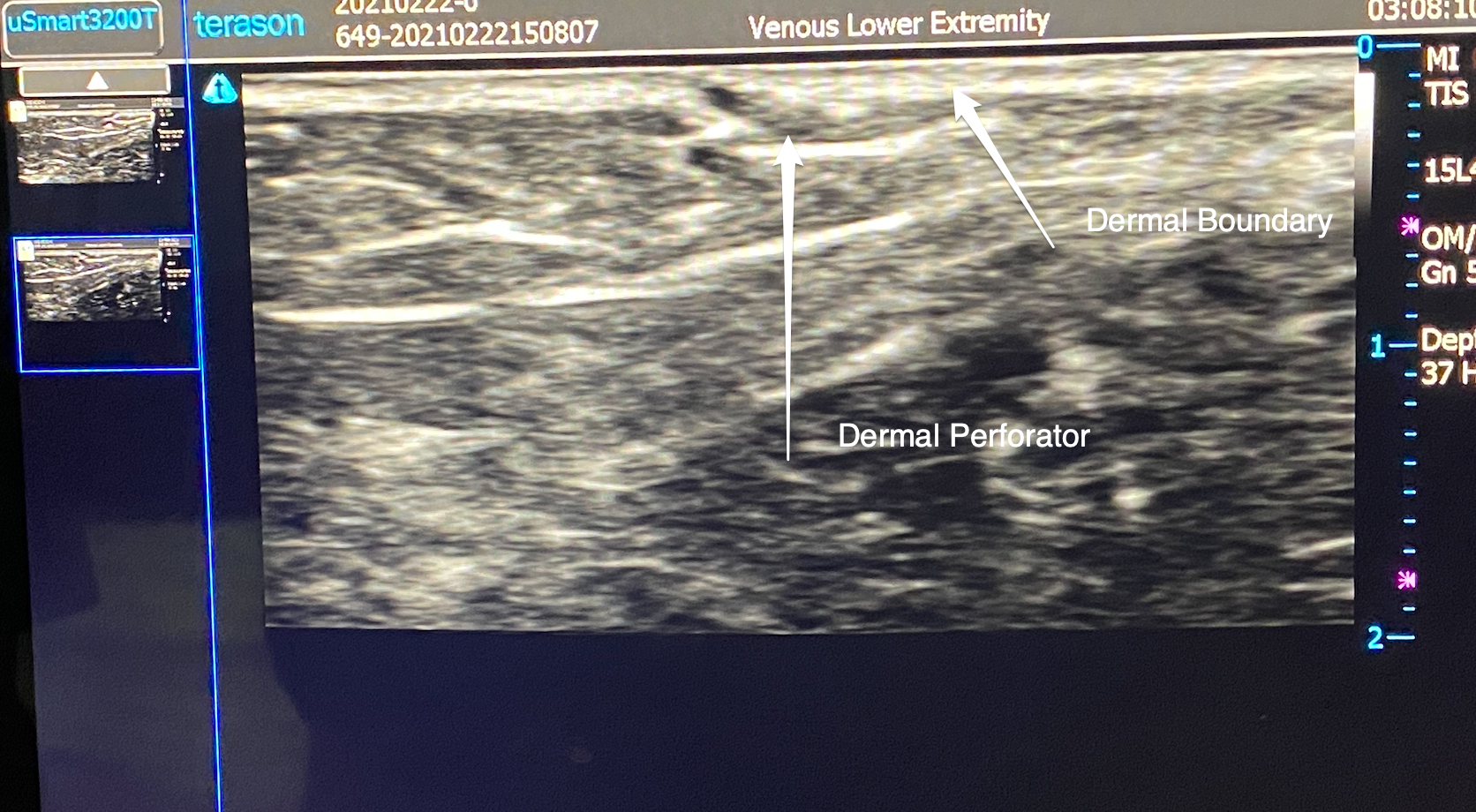
FIGURE 2 Ultrasound image of dermal perforator.
For sclerotherapy to be successful, enough damage to the vessel must occur without vessel wall perforation. Sclerosants are detergents and, as such, can bind to the intimal layers and cause protein denaturation. When this happens, intimal disruption and endothelial loss occur.
Depending on sclerosant strength and time of contact, smooth muscle wall alterations can happen, as well as wall disruption. Histologically, intimal disruption appears as a ragged lining of the vessel wall. There may be intraluminal cellular debris, which can cause early vessel thrombosis. Smooth muscle alterations may appear as a transformation of the smooth muscle or thinning of the vessel wall (Fig. 3). Loss of vessel wall integrity allows erythrocytes to penetrate the dermal tissues, causing an intense inflammatory response and, eventually, diffuse hemosiderin deposition or, in many cases, angiogenesis.
FIGURE 3 One millimeter spider vein treated with Sotradecol 0.1% 11⁄22 NS.
Thus an ideal sclerosant concentration will cause enough damage to elicit vessel fibrosis but not be strong enough to cause vessel wall disruption. The two FDA approved sclerosants currently in use are Sodium Tetradecyl Sulfate (STS) and Polidocanol. Compounded drugs are not used. Polidocanol is nonionic while Sotradecol is ionic. Polidocanol can be diluted with normal saline (NS) or bacteriostatic water, and its potency is unaffected. The ionic detergent STS is very much affected by method of dilution. Histology shows a threefold increase in sclerosant activity when diluted with bacteriostatic water. This phenomenon occurs due to the critical micelle concentration (CMC) inherent in all detergent molecules. Normal saline will bind with ionic components of STS and lower the CMC. Thus the potency of the sclerosant is less. The chemical interaction is more complex than stated here, but simply put, Sotradecol 0.1% compares to Polidocanol 0.3% when saline is used as a diluent. When bacteriostatic water is the diluent, STS 0.1% is comparable to Polidocanol 0.5%.
Using STS diluted with bacteriostatic water may yield faster clearance, but potential sequelae such as staining may occur. However, most staining is transient and in most cases resolves within three months. The breakdown of hemoglobin to hemosiderin is the etiology of staining. The macrophage response is the factor in the duration of staining. The more intense the inflammation, the more resident dermal and circulating macrophages are recruited. Once macrophage phagocytosis occurs, staining may persist for prolonged periods (Fig. 4).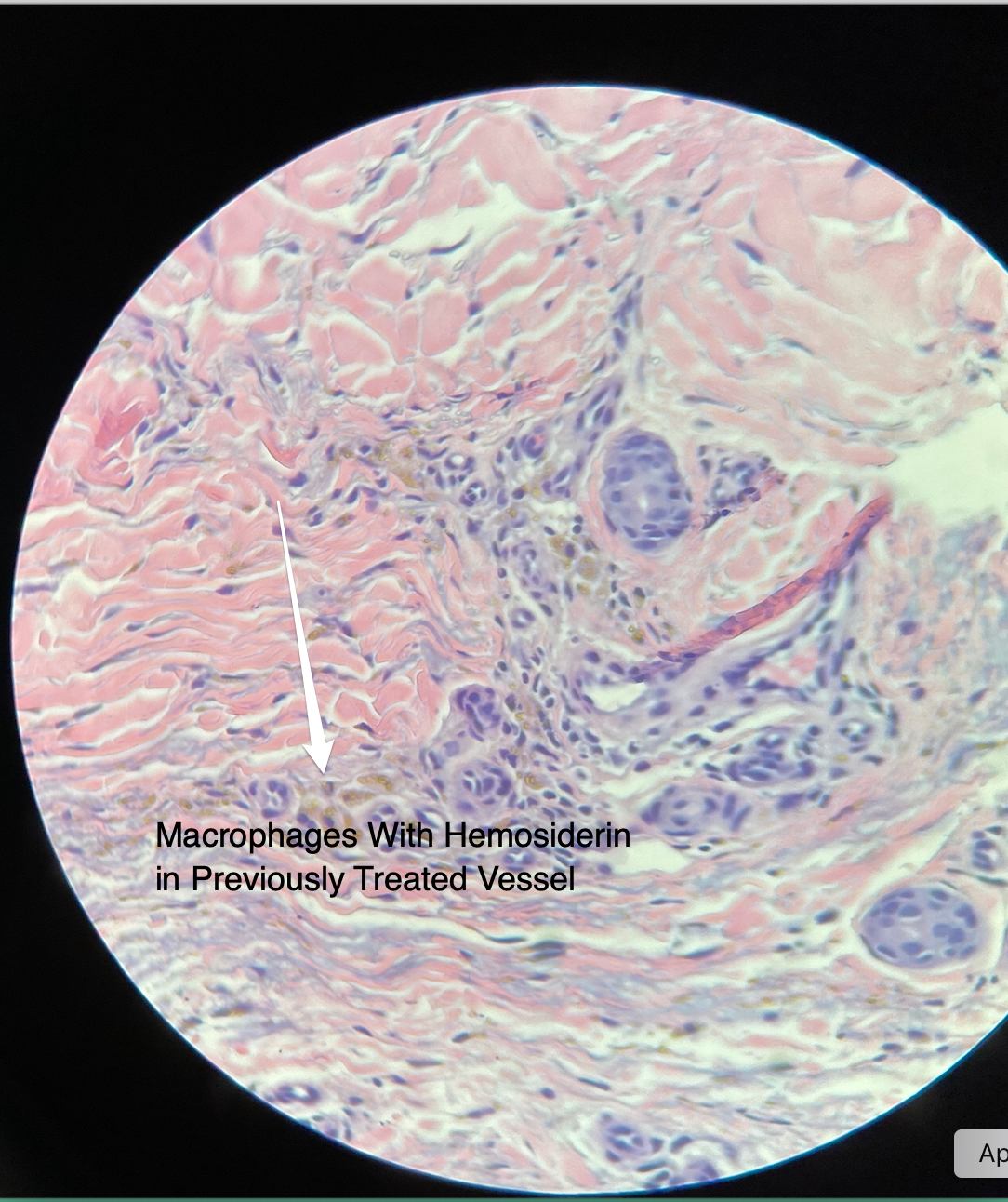
FIGURE 4 Macrophages with hemosiderin in previously sclerosed spider vein.
Each patient will have some variability in response. If possible, do a test injection on the patient with the concentration of sclerosant with which you are comfortable and vary the concentration as needed.
However, there are more factors that must be considered besides sclerosant type and concentration. A test injection might prove useful for scattered and somewhat isolated telangiectasias. For the more common grouped complexes, preliminary testing is useless (Fig. 5). The major cause of sclerotherapy failure is venous refilling, both antegrade and retrograde.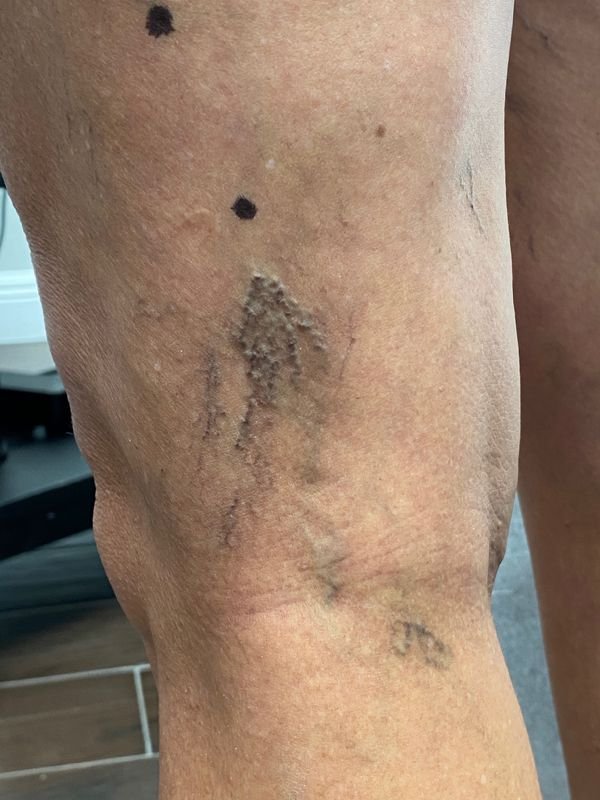
FIGURE 5 Telangiectasia complex of lateral reticular system
The step before injecting the sclerosant is to minimize retrograde filling. This technique involves subdermal tumescent infusion. Ramlet, 2012 first proposed this technique for the treatment of angiogenesis. Subdermal tumescent reduces the size of the vessel lumen and diminishes retrograde filling from the vast cutaneous venous network (Fig. 6). Make sure that a small portion of the complex does not have subdermal tumescent infusion, or, in five minutes, there may be no visible veins to inject. Subdermal tumescent is not used in the area of the ankle or anterior lower leg.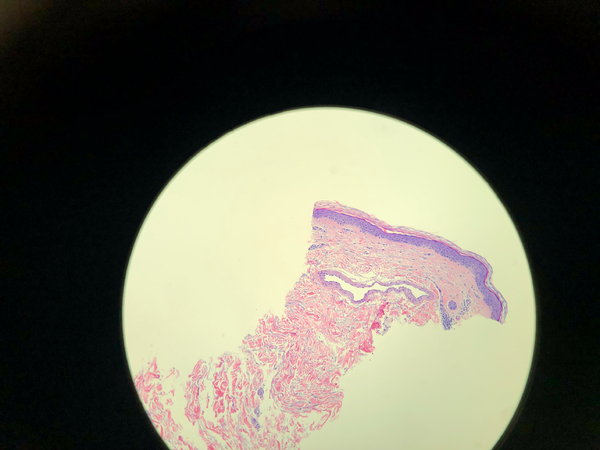
FIGURE 6A Telangiectasia prior to subdermal tumescent.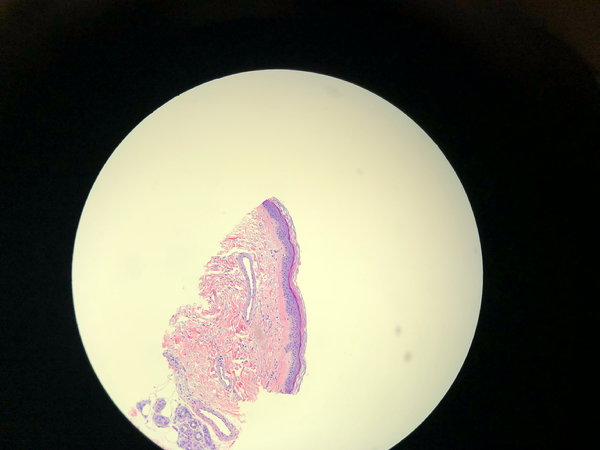
FIGURE 6B Note volume decrease post subdermal tumescent.
The second step is to inject the sclerosant. I found that Sotradecol 0.1% foam or Polidocanol 0.25% foam is ideal for one millimeter telangiectasia as foam causes apposition in many patients’ vessel walls (Fig. 7).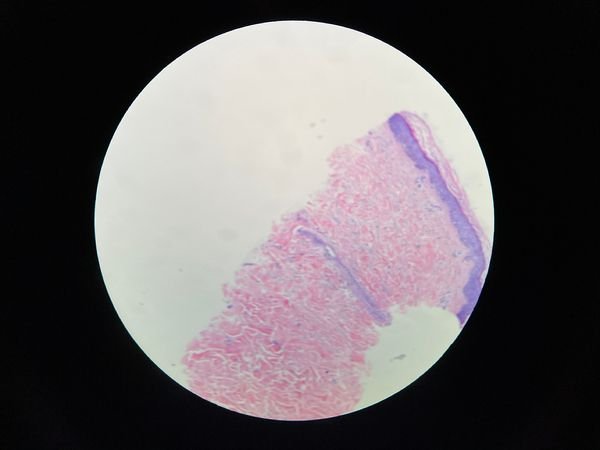
FIGURE 7 Subdermal tumescent followed by foam injection using Sotradecol 0.1%. Note apposition of vein wall.
Thus to obtain the best results, you must pick an appropriate sclerosant concentration and minimize antegrade and retrograde filling of the treated complex. Antegrade filling, as mentioned before, occurs from the dermal perforator. My preferred technique is to use a one millimeter dermal punch at the site of the perforator. This is the third step.
This site is easily identified as the focal point from which the complex originates (Fig. 8). A small vein hook can also be inserted to avulse the underlying reticular vein. In selected cases, the punch specimens were cut at four-micron levels and stained with haematoxylin and eosin. The telangiectasia in the punch specimen could be analyzed for histological determination of sclerosant strength.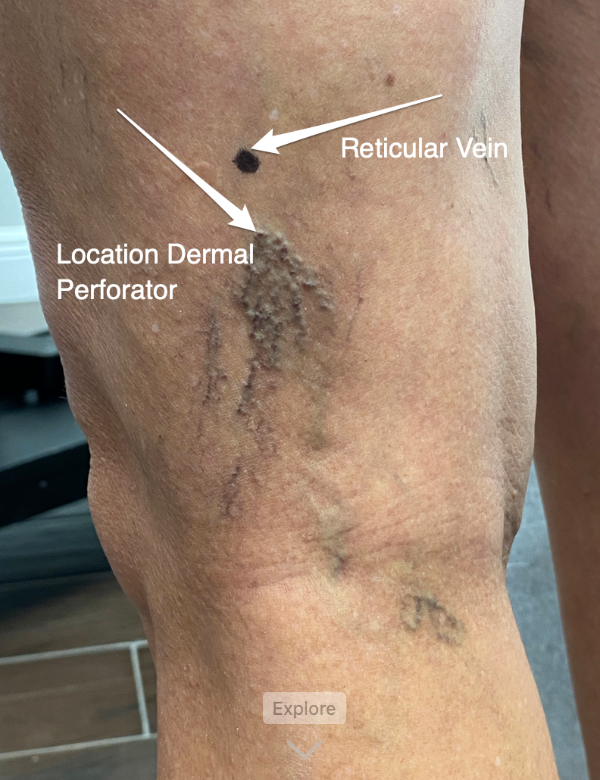
FIGURE 8 Location of dermal perforator.
The fourth step is to flush the complex if early clotting is noted. Early clotting presents as grayish or blackish color to the treated vessels. Flushing is done by injecting the distal telangiectasias with bacteriostatic water retrograde through the punch site.
The last step is to compress the area with a pool noodle, cut and wrapped in cast padding. This compression is fixed in place and remains on for at least 48 hours. After treatment, local compression is essential for a good outcome (Fig. 9). This technique prevents refilling after tumescent becomes ineffective. It cannot be stressed enough how important this step is in obtaining good results. The longer the local compression, the better the outcome. Local compression prevents refilling of the treated complex, minimizing intravascular clotting of the telangiectasia.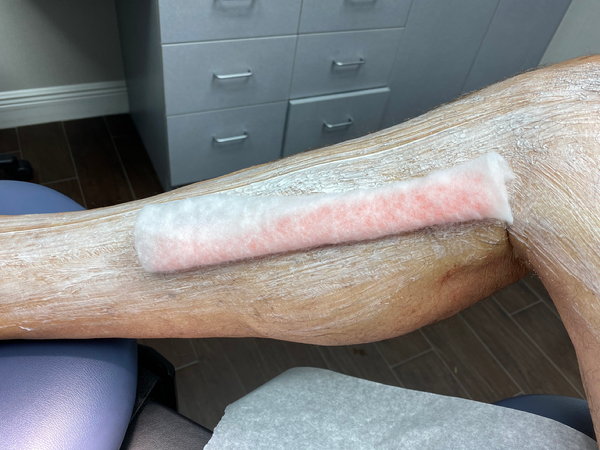
FIGURE 9 Example of post-procedure compression with pool noodle wrapped in cast padding.
In some cases, additional micro incisions are made to decrease the incidence of thrombi in the complex after treatment and before compression. More detailed descriptions and videos of these procedures are available online at aestheticveintraining.com.
After removing the compression, we recommend Dermaka® (a natural plant extract cream) to reduce bruising and inflammation. The cream is applied two to three times a day until bruising has cleared. At two weeks, the treated sites are examined. If present, trapped blood is removed by flushing or microincision.
All patients are made aware of the treatment protocols we recommend as they differ from ordinary sclerotherapy. Informed consent must be obtained, and the patient must understand the importance of the post-treatment protocol.
Conclusion
In conclusion, for the best results for telangiectasia complexes, use a five-step approach.
- Inject tumescent subdermally under the complex—not
- Inject Sotradecol 1% or Polidocanol 0.25% foam retrograde.
- Punch excision of the dermal
- Flush with bacteriostatic water if needed, retrograde through the punch
- Apply local compression for two to three days with foam such as a pool noodle wrapped in cast padding, and follow recommended post treatment
If subdermal tumescent and foam is not performed, use either Sotradecol 0.075% to 0.1% ½ NS or Polidocanol 0.3%. At these concentrations, excellent results can be obtained as long as the inflow is addressed. However, more treatment sessions may be necessary. All of our treatment recommendations are based on extensive histological studies.
The concentrations recommended produce the desired luminal changes with minimal adverse sequelae based on numerous histological studies.
As a vascular surgeon working in one of the largest dermatology practices in the United States, I have had the opportunity to do histology studies in the dermatopathology lab. I have come to realize that there is limited research on telangiectasias. My treatment approaches have dramatically changed in the last few years. Understanding the pathophysiology of CVH is essential.
I would like to thank Dr. Ted Schiff and Dr. Jackie Russo, as well as Carmen Minni, Lab Supervisor, at Water’s Edge Dermatology, Palm Beach Gardens, Florida for their invaluable assistance in preparing and interpreting our many studies.
Ronald Bush, MD, FACS
Ronald Bush, MD, FACS, is one of the nation’s foremost specialists in venous disease. He has published numerous peer reviewed journal articles and is an innovator of many techniques utilized in the treatment of venous disease. He is board-certified in vascular surgery.
Dr. Bush trained at Indiana University School of Medicine and Walter Reed Army Medical Center in cardiothoracic surgery. For the past 20+ years, Dr. Bush has devoted his practice solely to the treatment of venous disease. Areas of research currently are devoted to the manifestations of cutaneous venous hypertension. Dr. Bush has developed a targeted approach to the treatment of venous ulcers and has published his technique in various journals. He does histological research on the histology of venous disease at Water’s Edge Dermatopathology Lab, Palm Beach Gardens, Florida. He recently co-authored a book entitled A Guide to Treating Face Veins.
References:
Bush R, Bush P. Evaluation of sodium tetradecyl sulfate and polidocanol as sclerosants for leg telangiectasia based on histological evaluation with clinical correlation. Phlebology 2017;32(7):496-500.
Bush R, Bush P. Response to Professor Whitely, concerning “Evaluation of sodium tetradecyl sulfate and polidocanol as sclerosants for leg tel- angiectasia based on histological evaluation with clinical correlation. Phlebology 2018;33(3):215-216.
McAree B, Ikponmwosa A, Brockbank K, Abbott C, Homer-Vanniasinkam S, Gough MJ. Comparative stability of sodium tetradecyl sulphate (STD) and Polidocanol Foam: Impact on vein damage in an In-vitro Model. Eur J Vasc Endovascular Surg. 2012;43(6):721-725. Parsi K. Interaction of detergent sclerosants with cell membranes. Phlebology 2015;30(5):306-315.
Ramelet A. Sclerotherapy in tumescent anesthesia of reticular veins and telangiectasias. Dermatol Surg. 2012;38(5):748-51l. Santos FKG, Neto ELB, Mourna MCPA, Dantas TNC, Neto AAD. Molecular behavior of ionic and nonionic surfactants in saline medium. Colloid Surf A. 2009; 333: 156-162.
Whiteley M S, Dos Santos S J, Fernandez-Hart, TJ. Media damage following detergent sclerotherapy appears to be secondary to the induction of inflammation and apoptosis: An immunohistochemical study. Eur J Vasc Endovascular Surg. 2016;51(3):421-428.
Wong K, Chen T, Connor D, Behnia M, Parsi K. Basic physiochemical and rheological properties of detergent sclerosants. Phlebology. 2015; 30(5):339-349.


