 Venous ulcers are a manifestation of chronic venous insufficiency. Up to 2.7% of the Western population has active venous ulcers and a history of venous ulcers that have healed1. The morbidity associated with venous ulcers continues to impose an enormous cost on health care systems2,3.
Venous ulcers are a manifestation of chronic venous insufficiency. Up to 2.7% of the Western population has active venous ulcers and a history of venous ulcers that have healed1. The morbidity associated with venous ulcers continues to impose an enormous cost on health care systems2,3.
Incompetent perforator veins (IPV) have long been associated with venous disease and ulceration. Deciding when and how to treat these IPVs can be a difficult problem. Perforator veins in and around the ankles are particularly vulnerable to incompetence, and the pressure buildup from the reflux of blood increases the likelihood of edema, skin discoloration, and ulceration. IPV treatment thus, has become a regular procedure to lower venous hypertension and improve ulcer healing. Compression therapy has long been a cornerstone in the treatment of venous incompetence and reflux. Due to poor ulcer healing rates with compression alone, other treatment strategies have been geared toward treating perforator incompetence to improve ulcer healing and decrease recurrence4, 5.
Guidelines from the American Venous Forum and Society for Vascular Surgery recommend treatment of pathologic perforator veins (PPV) when they are > 3.5 mm in size, demonstrate reflux of > 500 msec, and occur in the vicinity of an active or healed ulcer or at the site of skin damage (CEAP 4b – CEAP 6 classification). Historically IPVs were treated with open surgery, which presented many wound healing issues. Current AVF/SVS guidelines recommend that PPVs now be ablated by percutaneous techniques such as sclerotherapy, radiofrequency ablation, or laser ablation6. These recommendations reflect a shift from open surgery to minimally invasive ablative therapies due to excellent technical success and few complications7.
Percutaneous closure of PPVs is simple and safe. Procedures are generally performed in the office with oral sedation if any. In my experience, the 400 Micron Perforator & Accessory Vein Ablation Kit* (PVAK (figure 1); AngioDynamics, Inc.) provides the easiest and most effective means to perform perforator ablation.
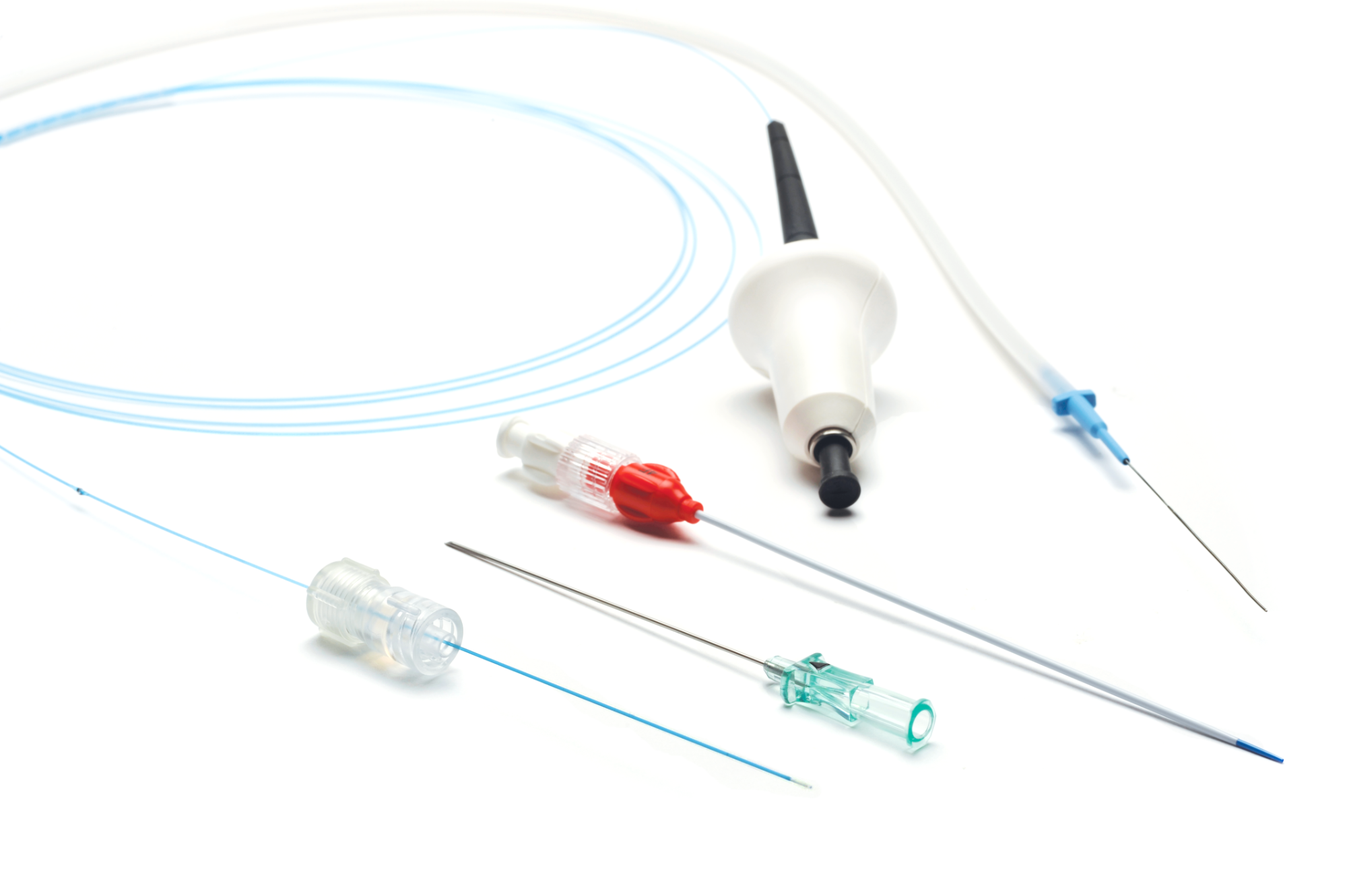
Figure 1: Kit components
In my use of PVAK, I image the perforator vein in the longitudinal axis and puncture the PPV with a micro-puncture needle. Through this, I feed the 400-micron laser fiber and position the tip at or slightly below the point of fascial crossing, depending on the anatomy. This manner of perforator cannulation can be performed without the assistance of a tech. The direct placement of the laser fiber through the needle (Figure 2), without the need for wire exchange, limits spasm of the PPV. The laser tip is easy to see and position. After the laser fiber is in position, I infiltrate more local anesthesia around the targeted vein, making sure to infiltrate below the PPV to push it away from the deep vessels. I then activate the laser at the setting of 6 watts, and deliver 100 joules for the first treatment point, and then 50 joules every 2 mm as I back out of the PPV, stopping all energy about 1 cm from the puncture site to avoid the possibility of a skin burn. It takes under 1 minute for the energy to be delivered. With this technology, a PPV can be closed with a 21-gauge needle puncture.
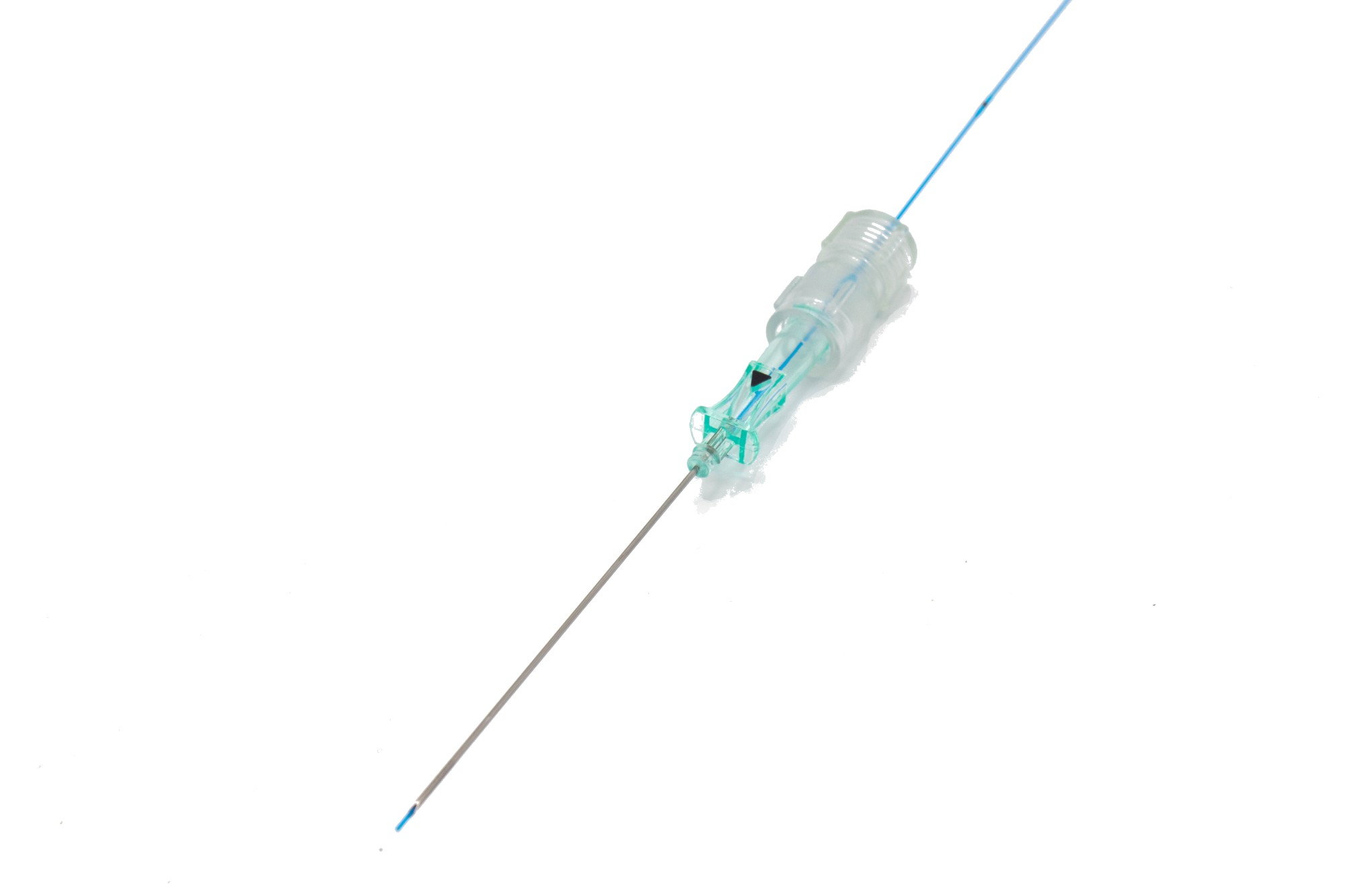
Figure 2 : Needle and fiber being “one” system
Other percutaneous modalities to close perforator veins include radiofrequency ablation and sclerotherapy. Radiofrequency ablation requires a 7 French introducer and the stiff ablation catheter, which can be difficult to pass through tortuous or spastic veins. In addition, radiofrequency requires 30-60 seconds of energy in 4 quadrants, every 3 mm along the perforator veins. This can result in an ablation lasting for as long as 10-12 minutes. In CEAP 6 patients with mobility issues, this long treatment time can be very uncomfortable. Sclerotherapy, usually using physician-compounded foam as an off-label use of FDA-approved sclerosants, is an easy means to try to close the pathologic perforator veins. Unfortunately, ultrasound-guided foam sclerotherapy (UGFS) is only effective in closing PPVs 50-60% of the time, a significantly lower rate than thermal ablation5,8,
Adverse events around perforator closure are rare. Deep vein thrombosis, usually affecting tibial and muscular veins, is very low5. Other potential events around PPV treatment can include failure to close and remote recanalization. Nerve or skin damage is possible but extremely rare7.
The SeCure study 9,10, 11 evaluated the safety and effectiveness of the 400 Micron Perforator & Accessory Vein Ablation Kit for the treatment of IPVs. This was a single-arm, prospective, multi-center, non-blinded clinical trial with a sample size of 83 patients (125 treated perforator veins). The study enrolled patients with CEAP class 4b, 5 and 6. The primary endpoint was "Acute Primary Ablation Success," defined as complete lack of flow or IPV disappearance in the entire treated segment, as measured via duplex ultrasound imaging performed 10 days (+/- 3 days) post-procedure, and secondary endpoints were: technical success, 1, 3, 6, 9 and 12-month primary ablation closure rates; changes in revised Venous Clinical Severity Score (rVCSS), quality of life, ulcer healing and adverse events.
The results of the SeCure study demonstrated a success rate of 76.8% for acute primary ablation success rate after the 10-day visit. This was statistically significant (p < 0.05) from the performance goal of 70%, which had been taken from radiofrequency ablation literature. Successful primary ablation was achieved in 1, 3, 6, 9, and 12-month follow-ups. At each time point, the mean rVCSS and the quality of life (VEINES-QoL) were significantly improved from baseline (p < 0.05), with ulcer healing presenting promising trends. Device-related adverse events were rare. Moderate pain, small posterior tibial deep vein thrombosis, and chronic venous obstruction were noted. There was one death, but this was not associated with the treatment.
The SeCure study is unique in that it is the only multicenter trial that has isolated the effect of perforator ablation on the patient's quality of life and ulcer healing. The > 95% technical success rate speaks to the ease of use of this technology. The high closure rate, coupled with a low incidence of adverse events, illustrates the device’s clinical success. Based on the positive results of this rigorous study, the 400-micron fiber received 510(k) clearance for an expanded indication and is currently indicated for use in the treatment of Superficial vein reflux of the greater saphenous vein associated with varicosities, in the treatment of incompetence and reflux of superficial veins in the lower extremity and for the treatment of incompetent (i.e. refluxing) perforator veins (IPVs).
The 400 Micron Perforator & Accessory Vein Ablation Kit from AngioDynamics is an FDA-approved technology to treat IPVs in patients with ulceration or skin damage. This system, which allows for treatment through a single 21-gauge needle puncture, is safe, comfortable, and the most successful way to close pathologic perforator veins.
Important Risk Information
Indications for Use: The VenaCure EVLT 400 μm Perforator and Accessory Vein Ablation Kit is intended for use in the treatment of superficial vein reflux of the greater saphenous vein associated with varicosities.
The VenaCure EVLT 400 μm Perforator and Accessory Vein Ablation Kit is indicated for the treatment of incompetence and reflux of superficial veins in the lower extremity and treatment of incompetent (i.e., refluxing) perforator veins (IPVs).
Contraindications: Contraindications include but are not limited to the following, Patients with thrombus in the vein segment to be treated. Patients with an aneurysmal section in the vein segment to be treated. Patients with peripheral arterial disease as determined by an Ankle-Brachial Index < 0.9. Patients with an inability to ambulate. Patients with deep vein thrombosis (DVT). Patients who are pregnant or breastfeeding. Patients in general poor health. Other contraindications may be raised by the individual physician at the time of treatment.
Extremely tortuous vein segments may require treatment by alternative techniques (phlebectomy, sclerotherapy).
Warnings:
- Contents supplied STERILE using an ethylene oxide (EO) process. Do not use if the sterile barrier is damaged. If damage is found, call your sales representative. Inspect prior to use to verify that no damage has occurred during shipping.
- For single patient use only. Do not reuse, reprocess, or resterilize. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or lead to device failure, which, in turn, may result in patient injury, illness, or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient.
- After use, dispose of product and packaging in accordance with hospital, administrative, and/or local government policy.
- Treatment of a vein located close to the skin surface may result in a skin burn.
- Paresthesia may occur from thermal damage to adjacent sensory nerves.
- Tissue not targeted for treatment must be protected from injury by direct and reflected laser energy with appropriate eye and protective wear for any person present in the operating room.
- Prior to and during use, avoid damaging the fiber by striking, stressing, or excessive bending. Do not coil the fiber tighter than a radius of 60 mm.
- The positions of the Site Marks on the EVLT fiber have been matched to the introducer sheath provided in the VenaCure EVLT 400 μm Perforator and Accessory Vein Ablation Kit. Alternative sheaths must not be substituted.
- Do not tighten the compression clamp on sheath until fiber is in position.
- Laser protective eye wear must be worn by everyone in the treatment room, including the patient.
Adverse Events:
Potential complications include, but are not limited to the following: DEHP Exposure, Deep Venous Thrombus, Hematoma, Hemorrhage, Infection, Necrosis, Neovascularization, Non-Target Irradiation, Paresthesia, Phlebitis, Pulmonary Embolism, Skin Burns and Pain, Infection, Skin Pigmentation Alteration, Thrombophlebitis, Thrombosis, and Vessel Perforation.
Refer to Directions for Use and/or User Manual provided with the product for complete Instructions, Warnings, Precautions, Possible Adverse Effects, and Contraindications before the use of the product.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
AngioDynamics and VenaCure are trademarks and/or registered trademarks of AngioDynamics, Inc., an affiliate or subsidiary. All other trademarks are the property of their respective owners. ANGM 1170 US Rev 01 09/2019
References:
- Meissner MH, et al. Primary Chronic venous disorders. J Vasc Surg. 2007; 46 (Suppl S): 54S-67S.
- Heit JA, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg. 2001; 33(5): 1022-7.
- Olin JW, et al. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med. 1999; 4(1): 1-7.
- Ibegbuna V, et al. Haemodynamic and clinical impact of superficial, deep, and perforator vein incompetence. Eur J Vasc Endovasc Surg. 2006; 31(5): 535-41
- Kiguchi MM, et al. Factors That Influence Perforator Thrombosis and Predict Healing: Perforator Sclerotherapy for Venous Ulceration Without Axial Reflux. J Vasc Surg Venous Lymphat Disord. 2015 Jan; 3(1):125.
- O'Donnell TF Jr et al. Society for Vascular Surgery; American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. J Vasc Surg. 2014 Aug; 60(2 Suppl):3S-59S
- Dillavou ED, et al. Current state of the treatment of perforating veins. J Vasc Surg Venous Lymphat Disord.2016 Jan; 4(1):131- 5
- Hager ES, et al. Factors that influence perforator vein closure rates using radiofrequency ablation, laser ablation, or foam sclerotherapy. J Vasc Surg Venous Lymphat Disord. 2016 Jan; 4(1):51-6.
- Gibson K, et al. A Prospective Safety and Effectiveness Study using the endovenous laser ablation technique with a 400 μm optical fiber (SeCure trial). Presented at: American College of Phlebology meeting. 2018 Nov 8; Nashville, TN.
- 10.Adelman M et al. The SECURE Trial: Update on Perforator Ablation (Safety and Effectiveness Study: VenaCure Endovenous Laser Treatment (EVLT). Presented at: VEITH meeting. 2018 Nov 15; New York, NY.
- Elias S, et al. The SECURE Trial: Update on Perforator Ablation (Safety and Effectiveness Study: VenaCure Endovenous Laser Treatment. Presented at: International Vein Congress meeting; 2019 Apr 25; Miami, FL.
* The SeCure study was sponsored by AngioDynamics, Inc. Dr. Kathleen Gibson (Vascular Surgeon, Lake Washington Vascular, Bellevue, WA) was the Principal Investigator of the SeCure study.
The study data was presented at the American College of Phlebology meeting (6-8 November 2018, Nashville, TN); VEITH symposium (13–17 November 2018, New York, NY) and the International Vein Congress (25-27 April 2019, Miami, FL). The manuscript from this study is currently under peer review.
**Dr. Ellen Dillavou is a paid consultant of AngioDynamics, Inc.
*** Views and opinions expressed in the article are of the author and do not necessarily reflect the views and opinions of AngioDynamics, Inc.
**** Photographs courtesy of AngioDynamics, Inc.


