
Acute DVT, PTS and Streptokinase
It is well established that patients who present with acute deep vein thrombosis (DVT) of the lower extremity have a high prevalence of developing post thrombotic syndrome (PTS) if treated with anticoagulation alone. The incidence varies from one publication to another, but a recent publication by Kahn et al. put the number at 43 percent of all comers.1
It has also been demonstrated that patients with more proximal lower extremity DVT, namely of the iliac and common femoral veins, have significantly higher risk of developing symptoms and signs of the PTS than those with a more peripheral thrombosis.1 It was recognized early on that those patients who had early resolution of the thrombus would fare better from the standpoint of PTS compared to those with less or no thrombus resolution.
These observations were made in the era of systemic delivery of Streptokinase, which at that time was used for myocardial infarction and pulmonary emboli. Many smaller randomized studies were conducted for acute DVT comparing systemic delivery of streptokinase in addition to anticoagulation versus just anticoagulation alone. In brief, those studies showed less postthrombotic venous changes on follow-up (in most cases venographic followup) and less clinical findings of the PTS for patients treated with systemic streptokinase.2 Due to high prevalence of what was considered serious bleeding complications, this treatment option fell in disfavor.3
Catheter Directed Thrombolytic Therapy
In the late 1980s, catheter directed thrombolytic (CDT) therapy had found its way into management of peripheral arterial thromboembolic disease. This promised lower bleeding risk (lower dose of drug) and higher efficiency over systemic delivery.
The first case report of using catheter directed thrombolysis for iliofemoral DVT was published in 1991.4 Several larger case studies followed including a large registry with over 400 patients from the United States, all of which seemed to demonstrate satisfactory safety profile and efficiency.5,6
From those studies spun quality of life (QOL) comparative studies that demonstrated in a non-randomized way better QOL and better life satisfaction in cohorts treated with CDT compared to patients with similar distribution of DVT but treated with anticoagulation (AC) alone.7 This predated any usage of mechanical devices which were intended to either mechanically break down the thrombus, aspirate the thrombus or a combination of those two.
The treatment time by simply using CDT alone was long, typically around three days. With the addition of mechanical thrombectomy (MT) the average treatment time was cut to much less, typically around 30 hours or even less. With the use of mechanical thrombectomy, no additional CDT was needed in four percent of cases according to the PEARL registry.8
Registries and Studies on DVT and PTS
The early registries on CDT for DVT reported high utilization of endovascular stents, 33 percent and 45 percent in the two largest ones. Stents were placed in almost all instances to treat underling outflow lesions in the iliac veins or residual thrombus which had not responded to the thrombolytic therapy or mechanical thrombectomy.5,6
The medical community does look at randomized studies as the ultimate proof of effectiveness and safety. CDT and MT suffered from lack of such evidence.
Early on, there were several small, randomized studies on the use of systemic thrombolysis for DVT. Those were not large enough to reach statistical significance and it was ultimately meta-analysis that demonstrated the effectiveness of this approach but also demonstrated the unacceptable high bleeding risk.
The first randomized study on CDT came from Egypt in 2002.9 Even though the authors only included 36 patients in this study comparing CDT followed by anticoagulation to that of anticoagulation alone, they found significantly higher venous patency and less valvular reflux at six months in the CDT group.9
It was then in 2009 that the first report from the Norwegian CaVenT trial was published. The first publication was on a six-month follow-up after evaluation of the first enrolled 130 patients who were randomized to CDT versus anticoagulation alone (53 patients versus 50 patients respectively). The short follow-up found significantly better iliofemoral patency and less obstruction in the CDT group, but disappointingly no difference in femoral vein insufficiency.10
In 2012 the same group reported on the study, now having enrolled 209 patients (101 patients in CDT and 108 in anticoagulation alone). At the 24-month followup there was significant reduction in PTS. There were 20 bleeding complications, three of which were considered major complications, one abdominal wall hematoma, one calf compartment syndrome and one inguinal hematoma.11 The results were not felt to be as substantial as one had hoped for and criticisms were raised in order to understand the perceived shortcoming.12
The critics pointed out more extensive iliac vein involvement in the CDT cohort and lower than expected usage of endovascular stents among other things. Only 15 percent of the limbs were treated with stents for outflow obstruction. Finally, no mechanical thrombectomy devices were used, only direct infusion (CDT), and the treatment time was rather long at 2.4 days.12
In comparison, the American Venous Registry found that stents were used in 33 percent of the patients5 and the University of Minnesota publication6 reported stents to be used in 45 percent of treated limbs which had undergone CDT. From the CaVenT trial, a five-year follow-up was subsequently published in 2016. A more prominent difference was noted in PTS between the two groups in favor of those treated with CDT (number needed to treat four) but surprisingly this did not translate to a difference in quality of life between the two cohorts.13
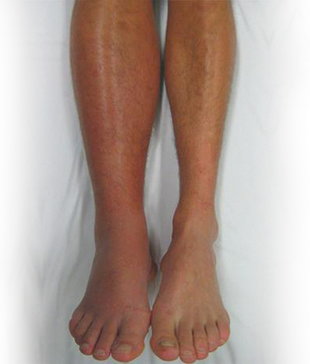
The ATTRACT Trial
This brings us to the topic of this short discussion, namely the ATTRACT trial. The name is derived from Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis. This is a multicenter, randomized (randomization is 1:1), open-label, controlled clinical trial with the primary hypothesis that CDT reduces the incidence of PTS in patients with acute lower extremity DVT by one-third over an observation time of two years.
The Villalta scale is used to assess the primary endpoint. The secondary outcomes include safety, general and venous disease-specific quality of life, relief of early pain and swelling and cost-effectiveness. The calculated number of patients needed was 692 and this enrollment goal was reached on December 14, 2014.
The two-year results would not be available until two years later and preliminary data were presented at the Society of Interventional Radiology meeting in Washington, DC, on March 6, 2017 by the Principal Investigator, Dr. Suresh Vedantham of the Mallinckrodt Institute of Radiology at Washington University School of Medicine.14
The Data
The preliminary data demonstrated that at 30 days there was less pain and limb swelling in the CDT group compared to the AC alone group, but recurrent venous thromboembolism was no different in the two groups, 12.5 percent for the CDT group versus 8.5 percent in the AC group (p=0.087). There was no difference in QOL from baseline at the 24-month follow-up between the two groups.
The encouraging data appears at the two year follow-up at which time the PTS, defined at its most general as Villalta score of five or more, was equal between the two groups, 46.7 percent for CDT and 48.2 percent for the anticoagulation alone group (p=0.56), but when using more stringent criteria of moderate to severe PTS 17.9 percent of the patients in the CDT group had PTS compared to 23.5 percent in the AC group (p=0.035).
If one looked only at the patients with iliofemoral DVT, and again using Villalta score of 10 and higher (moderate to severe PTS), these numbers were 18.4 percent versus 28.2 percent (p=0.016). This evaluation for the femoropopliteal segment revealed a much lower incidence of PTS and no statistical difference, 18.1 percent incidence for patients treated with AC alone at two years.
Major bleeding within 10 days was observed in 1.7 percent in the CDT cohort compared to 0.3 percent in the group treated with AC alone (p=0.049). The rate of any bleeding complications was 4.5 percent versus 1.7 percent in the CDT group compared to the AC group (p=0.034). There were no intracranial or fatal bleeding episodes.
Going Forward
So where does this leave us? Dr. Mark H. Meissner of the University of Washington Medical Center and his colleagues demonstrated that early thrombus resolution does preserve function of the valvular elements15 and venous obstruction is felt to be detrimental to the venous function, hence the “open vein” concept.16 Early experience using systemic thrombolysis clearly demonstrated venographic evidence of how effective that treatment is in lysing acute DVT, and at least one randomized study showed less PTS in patients treated with systemic streptokinase compared to AC alone at the six-and-a-half-year follow-up.17
Evidence is being built in support of early thrombus removal. The method used for thrombus removal has changed from a systemic thrombolytic delivery to a more local delivery, but still using generally acting thrombolytic agents that inherently introduce bleeding risks. We are bound to stay with the current way of removing the thrombus for the foreseeable future, and it is quite clear that CDT has a lower risk of bleeding than systemic approach (no comparable studies). The thrombectomy process can still be accelerated with use of mechanical “debulking” of the thrombus, leading to lesser or even no use of thrombolytic agents and thereby intuitionally reducing the risk of bleeding and cost.8
The evidence from the three randomized studies we have for CDT all point to a decrease in the incidence of PTS. The ATTRACT trial seems to indicate that iliofemoral DVT should be the anatomic distribution we should focus on, at least with the currently available technology and pharmaceuticals. A reduction of moderate to severe PTS by 10 percent, as indicated by the ATTRACT trial and keeping in mind the symptoms associated with that level of the Villalta scale, is significant for the affected patients and comes with a very reasonable safety profile.14 Complications, mostly bleeding risk, are of the utmost concern, but pulmonary emboli have been associated with CDT, and in the Venous Registry there were two deaths from pulmonary emboli and one cerebral bleeding associated with CDT.5 Neither the CaVenT or the ATTRACT trials reported pulmonary emboli or cerebral bleeding.
Catheter Directed Thrombolysis, Stents, and Anticoagulation
There is therefore nothing in the ATTRACT trial which should deter us from offering CDT for select patients with iliofemoral acute DVT. Unfortunately, the ATTRACT trial has only given us data from the first two years of follow-up, but the CaVenT trial demonstrated even stronger evidence in favor of CDT at five years than at two years.
Both the CaVenT and the ATTRACT trials used fewer endovascular stents than would be expected and the inclusion was heterogeneous, including a mixture of patients with iliofemoral and femoropopliteal DVT.
As far as treatment for isolated DVT of the femoropopliteal segment goes, the picture is not as clear. Giving what we now know and the technology we have, treatment of this segment without associated iliofemoral involvement should only be offered with the intention of collecting data for research purposes.
Summary
In summary, the ATTRACT trial corroborated the general feeling within the venous community that femoral popliteal DVT should be treated with anticoagulation for the time being and that in select patients (younger, ambulatory, minimal co-morbidities) CDT should be utilized when there are significant clinical signs and symptoms. If at all possible a five-year follow-up on these patients would be helpful.
References
- Kahn, S.R., et al., Determinants and Time Course of the Postthrombotic Syndrome after Acute Deep Venous Thrombosis. Annals of Internal Medicine, 2008. 149(10): p. 698-707.
- Comerota, A.J. and S.C. Aldridge, Thrombolytic Therapy for Deep Venous Thrombosis: a Clinical Review. CJS, 1993. 36(4): p. 359-364.
- Goldhaber, S.Z., et al., Pooled analyses of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am J Med, 1984. 76(3): p. 393-397.
- Okrent, D., R. Messersmith, and J. Buckman, Transcatheter Fibrinolytic Therapy and Angioplasty for Left Iliofemoral Venous Thrombosis. Journal of Vascular and Intrventional Radiology, 1991. 2(2): p. 195-200.
- Mewissen, M.W., et al., Catheter-directed Thrombolysis for Lower Extremity Deep Venous Thrombosis: Report of a National Multicenter Registry. Radiology, 1999. 211(1): p. 39-49.
- Bjarnason, H., et al., Iliofemoral Deep Venous Thrombosis: Safety and Efficacy Outcome during 5 Years of Catheter-directed Thrombolytic Therapy. J Vasc Interv Radiol, 1997. 8(3): p. 405-418.
- Comerota, A.J., et al., Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. Journal of Vascular Surgery, 2000. 32(1): p. 130-137.
- Garcia, M.J., et al., Endovascular Management of Deep Vein Thrombosis with Rheolytic Thrombectomy: Final Report of the Prospective Multicenter PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) Registry. Journal of Vascular & Interventional Radiology, 2015. 26(6): p. 777-785; quiz 786.
- Elsharawy, M. and E. Elzayat, Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg, 2002. 24(3): p. 209-214.
- Enden, T., et al., Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. Journal of Thrombosis and Haemostasis, 2009. 7(8): p. 1268-1275.
- Enden, T., et al., Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. The Lancet, 2012. 379(9810): p. 31-38.
- Hofmann, L.V. and W.T. Kuo, Catheterdirected thrombolysis for acute DVT. The Lancet, 2012. 379(9810): p. 3-4.
- Haig, Y., et al., Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol, 2016. 3(2): p. e64-71.
- Vedantham, S., The ATTRACT Trial. Presented at the Annual meeting of the Society of Interventional Radiology, Washington DC, March 6th, 2017.
- Meissner, M.H., et al., Deep venous insufficiency: The relationship between lysis and subsequent reflux. Journal of Vascular Surgery, 1993. 18(4): p. 596-608.
- Kuo, W.T., Optimizing Catheter-directed Thrombolysis for Acute Deep Vein Thrombosis: Validating the Open Vein Hypothesis. Journal of Vascular and Interventional Radiology, 2013. 24(1): p. 24-26.
- Arnesen, H., A. Hoiseth, and B. Ly, Streptokinase of heparin in the treatment of deep vein thrombosis. Follow-up results of a prospective study. Acta Med Scand, 1982. 211(1-2): p. 65-68.


