By Darren J. Wennen MBA*, Daniel Carlson MS, MBA*, and Thomas F. O’Donnell Jr., MD#
*Tactile Medical, Minneapolis, Minnesota
#Tufts Medical Center, Boston, Mass. and consultant Tactile Medical
Contact: TFOD at [email protected]
 Chronic lower-extremity ulcers, which affect as many as 4.5 million people in the United States, consume a large share of health-care resources and present a major challenge to wound care professionals.[1] Although the causes of these ulcerations are complex and multifactorial, most are related to chronic venous insufficiency (CVI).[2] Chronic edema in the lower-extremities – which, as will be illustrated -- is usually an expression of phlebolymphedema, a combination of venous and lymphatic dysfunction. This is common among advanced CVI patients. Phlebolymphedema predisposes to the development of cellulitis,[3] which not only is the most common cause of hospitalization and increased cost, but also portends a poor prognosis for venous leg ulcer (VLU) healing.[4] Conversely, effective phlebolymphedema treatment has been shown to improve VLU healing.[5]-[6]
Chronic lower-extremity ulcers, which affect as many as 4.5 million people in the United States, consume a large share of health-care resources and present a major challenge to wound care professionals.[1] Although the causes of these ulcerations are complex and multifactorial, most are related to chronic venous insufficiency (CVI).[2] Chronic edema in the lower-extremities – which, as will be illustrated -- is usually an expression of phlebolymphedema, a combination of venous and lymphatic dysfunction. This is common among advanced CVI patients. Phlebolymphedema predisposes to the development of cellulitis,[3] which not only is the most common cause of hospitalization and increased cost, but also portends a poor prognosis for venous leg ulcer (VLU) healing.[4] Conversely, effective phlebolymphedema treatment has been shown to improve VLU healing.[5]-[6]
Despite evidence supporting the treatment of lymphedema (LED) in CVI patients, including those at risk of or experiencing VLUs, venous leg ulcer management often ignores the lymphatic system.[7],[8] We believe this gap in care originates from the still commonly held belief that chronic edema in CVI patients can be resolved by addressing venous hypertension alone – a belief demonstrated to be incorrect.[9] In this editorial we highlight the growing body of evidence demonstrating the interconnected nature of the venous and lymphatic systems, discuss LED diagnostic and treatment options, and review recent evidence of outcomes and cost improvements that may result from providing early, effective lymphatic intervention to CVI patients with and without ulceration.
Origin of a misunderstanding: The modern pathophysiology of lymphedema
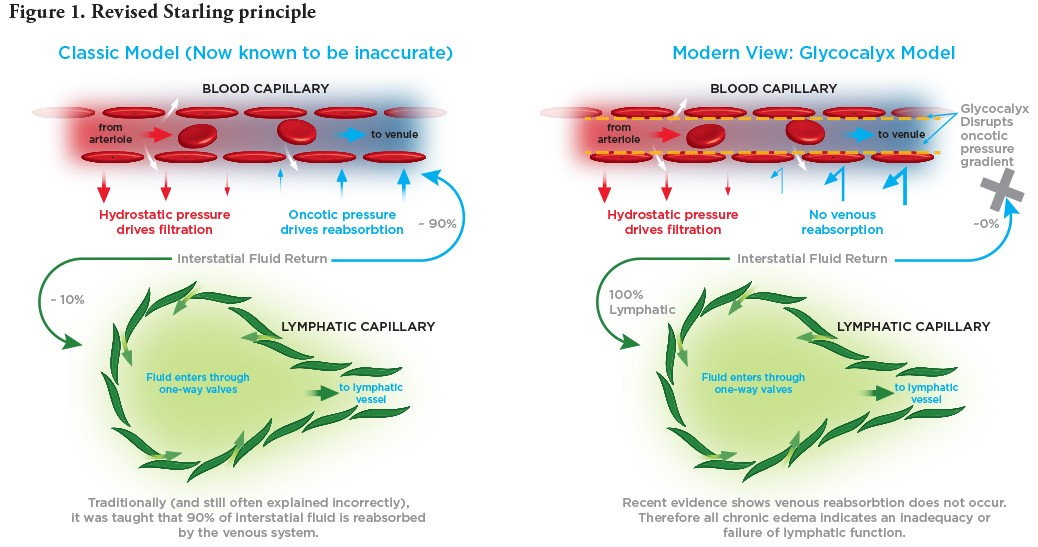
In a landmark paper published in 1896, physiologist Ernest Henry Starling[10] proposed that lymph fluids are removed from tissue spaces mainly through venous reabsorption. Starling deduced that transvascular fluid exchange depends on a balance between hydrostatic (pushing out) and oncotic (pulling in) pressure gradients, and that the capillaries and post-capillary venules behave as semi-permeable membranes reabsorbing fluid from the interstitial space. This paper established the “Starling principle” of venous reabsorption, a classic model still widely referenced.
In 2004, Adamson and colleagues[11] revealed that the effect of capillary oncotic pressure on transvascular fluid exchange is substantially less than predicted from the original Starling model. This discovery prompted a 2010 revision of the Starling principle by Levick and Michel11 based upon established evidence that capillaries push fluid into the interstitial space along their entire length, not just at the arteriolar-capillary junction. As noted by Mortimer and Rockson,3 the expected reabsorption of interstitial fluid via the venules simply does not occur; rather, interstitial fluid returns into the circulation only via the lymphatic system.
CVI and VLUs: the lymphatic connection
The revised Starling principle has significant implications for both venous and VLU care, as shown in Figure 1. In the U.S., an estimated 3 million people present with CEAP clinical class C 4 – 6 (advanced CVI).[12] Given the essential role of the lymphatics in fluid drainage, a singular focus on venous intervention for these patients may not resolve the chronic swelling secondary to CVI (phlebolymphedema) that most of them experience.8 As will be described, uncorrected phlebolymphedema can progress to a spiral of worsening complications that contribute to the development of VLUs, and may delay wound healing.
Phlebolymphedema begins as an accumulation of excess interstitial fluid, most commonly as a result of increased capillary pressure from venous hypertension.[13] Initially, the lymphatics compensate by increasing lymph drainage and thereby containing development of edema. However, this fluid excess eventually overwhelms the transport capacity of the lymphatic vessels, resulting in persistent swelling and increased risk of infection from the accumulation of pro-inflammatory (protein-rich) lymph fluid.3 Stasis dermatitis, a common inflammatory skin disease, is usually the first cutaneous sequela of phlebolymphedema.9 In the U.S., between 3 percent and 11 percent of the population experience edema and skin changes due to CVI.[14]
In CVI patients with stasis dermatitis, microangiopathic changes affecting small vessels occur in the lymphatic system as well as the venous system.[15] In biopsies from patients with CVI histologic examination shows structural lymphatic changes, including collapsed lumens, a disturbance of lumen-opening laments, and cellular interdigitations closing the lymphatic junctions. These disturbances ultimately lead to reduced lymphatic function.17 This functional reduction causes a buildup of protein-rich fluid, i.e., chronic edema, that can lead to a permanent immunocompromised district in the affected limb and a consequent spiral of worsening complications.[16] In addition to reducing transport capacity, recent evidence shows that lymphatic injury may also adversely affect immune response mechanisms (e.g., trafficking of antigen-presenting cells) within the endothelial layer of lymphatic vessels themselves.[17]
While the causes of VLUs are complex, VLUs are thought to be exacerbated by compromised local immunity.18 VLU healing can be impaired by local infection,4 which is closely associated with lymphatic failure. As Drs. Peter Mortimer and Stanley Rockson3 explain in their 2014 manuscript:
Lymphatics have an important immune surveillance function, as they represent the principal route of transport from tissues for antigen and immune cells. As such, lymphatics are important for adaptive immunity. Impaired lymphatic function predisposes to infection, which can clinically manifest as cellulitis/erysipelas, one of the most common medical conditions to present to hospital emergency departments.
The authors note that the lymph system filters and detoxifies lymph fluid of pathogens, damaged cells, cellular debris, viruses, bacteria and toxins; produces lymphocytes; and defends the body by directing specialized immune cells to fight against invading infections. VLUs are driven by the severity of venous disease but also exacerbated by the edema, skin changes, and recurrent cellulitis infections that are endemic to this patient population.[18] As stated by Eleonora Ruocco, Phlebolymphedema hampers the regular lymph flow and the normal trafficking of immune cells and initiates a typical instance of an immunocompromised district in the affected body region.18
In a 2016 study, Rasmussen et al.8 provided further insight into the lymphatic connection to VLUs. The objective of the study was to assess lymphatic function in subjects with venous leg ulcers using near-infrared fluorescence lymphatic imaging (NIRFLI) and to assess the impact of a single session with a pneumatic compression device (PCD) on lymphatic function. This study demonstrated that lymphatic function and anatomy were abnormal in all patients with CVI and VLUs compared with healthy subjects previously studied with NIRFLI. Rasmussen et al. not only consistently demonstrated “dermal backflow,” or stasis of contrast media within the lymphatics, in all lower extremities of VLU patients, but also showed lymphatic dysfunction in CVI patients with C0 through C4 disease, highlighting lymphatic impairment in the early clinical stages of CVI. Furthermore, in studies of normal subjects with high BMIs (approaching 40), they did not observe the localized dermal backflow seen in the VLU patients. This suggests that localized dermal backflow (i.e. lymphatic dysfunction) in the gaiter area could be part of the etiology of ulcer formation.
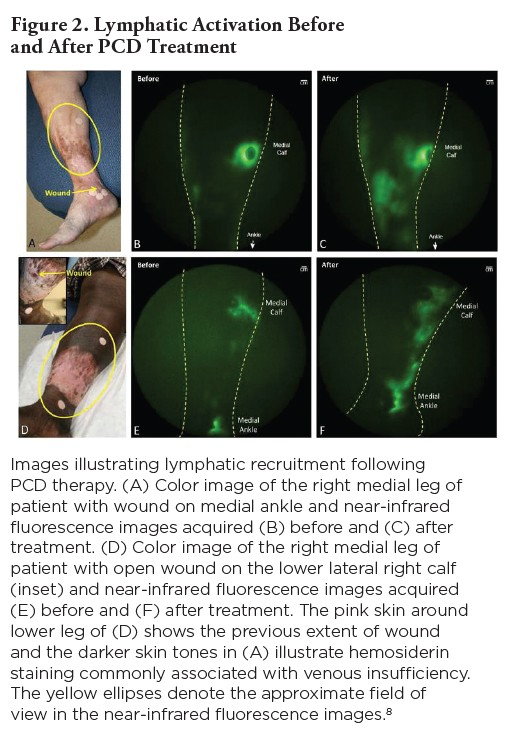
The study demonstrated that, while treatment of the underlying venous condition by reducing venous hypertension is important in resolution of VLUs, continued external stimulation of the lymphatics may also be necessary to improve symptoms stemming from permanent damage to the lymphatic system itself. Rasmussen et al. suggest that “once formed, an ulcer will not heal unless venous hypertension and the resulting excess capillary fluid filtration are ameliorated.” Individuals with severe venous obstruction may undergo interventional treatment to restore venous function and lower venous hypertension, thereby reducing excess capillary filtration and edema, but resolution of venous hypertension itself may not sufficiently resolve edema if lymphatic vessels have become dysfunctional. Rasmussen and associates point out that, despite the long-recognized lymphatic contribution to VLU formation and healing, recent authoritative reviews on CVI management did not define the lymphatic role in the outcomes of surgical treatment.
The lymphatic connection in CVI is reflected in the clinical sequelae of both CVI and phlebolymphedema, which closely resemble each other. As Eberhardt and Raffetto[19] wrote in 2014, an essential condition of advanced CVI is persistent ambulatory venous hypertension resulting in clinical sequelae that include pain, edema, skin changes, fibrosis and eventual ulcerations. These symptoms are often readily present in patients presenting with CVI-induced LED (phlebolymphedema).9,[20]
Phlebolymphedema complicates the clinical picture of CVI, triggering pain and discomfort, skin changes, and a higher risk of infection.3 Failure to correct lymphatic dysfunction paves the way for a spiral of negative complications including ulceration.18 This ultimately leads to increased physician office visits, costly treatments, and hospitalizations.[21] In the U.S., CVI may well be the most important predictor of LED development in the lower extremities.[22]
Epidemiology and economic impact
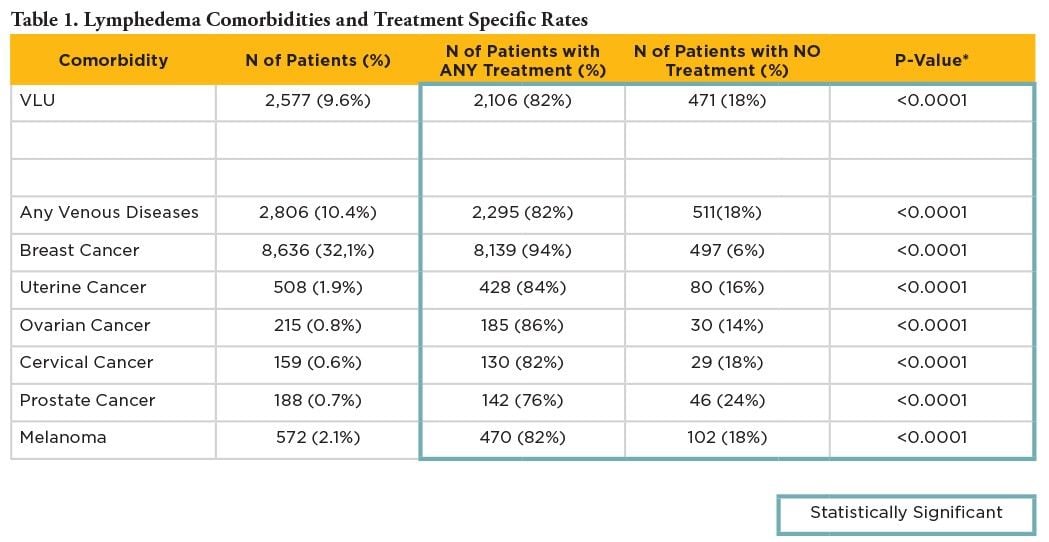
In an attempt to better define the causes of LED, Son et al.[23] conducted an analysis of de-identified HIPAA-compliant commercial administrative claims from the Blue Health Intelligence (BHI) Research Database (165 Million BC/BS members). Known comorbidities associated with LED were sought in the study sample of 26,902 patients diagnosed with LED who had been enrolled with continuous medical benefits for 12 months pre- and post-index date for the complete years 2012 through 2016. Breast cancer was the most frequently diagnosed co-morbidity with LED (32 percent), while other cancer types, including melanoma (2.1 percent) and prostate (0.7 percent), were less frequent. CVI patients, which were mainly (90 percent) comprised of patients with VLUs, were the second-leading comorbidity of LED and the most common non-cancer linked co-morbidity for LED (9.6 percent). It is important to note that these data are possibly subject to bias, as they rely on accurate physician identification and diagnosis of LED. Another analysis of BHI claims data showed that 90 percent of phlebolymphedema-diagnosed patients undergoing lymphedema treatment had VLUs, which suggests that recognition of phlebolymphedema may tend to be delayed until its later, more costly stages.24 As discussed above, current evidence suggests that most, if not all, CVI CEAP stage 3 and greater patients have lymphatic dysfunction (phlebolymphedema), which would make it the leading cause of LED in the U.S.
Son and colleagues’ analysis25 of BHI data also showed that even CVI patients with wounds received treatment for limb swelling (phlebolymphedema) less frequently than patients with breast cancer-related lymphedema (BCRL) (82 percent vs 96 percent, P < 0.0001), suggesting a treatment bias or gap. Whereas lymphedema is well-recognized by both physicians and patients as a complication of breast cancer treatment, recognition of the role of the lymphatics in CVI-related chronic swelling has historically been underappreciated.9 As a result, VLU treatment may continue to be focused on reduction of venous hypertension with insufficient attention paid to lymphatics.
VLUs are arguably the most severe expression of CVI and phlebolymphedema. Though epidemiology estimates vary, primary and secondary research suggests that the total annual incidence of VLUs in the U.S. is 600,000-700,000, which comprises approximately 20 percent of the CVI prevalence population.[24] In a study of 525 wound care centers of 154,644 patients with over 300,000 wounds treated from June 1, 2008 to June 30, 2012, Wilcox and associates[25] demonstrated that VLUs were the type most commonly treated. A venous etiology accounted for over 25 percent of the wounds, while diabetic foot ulcers were the second most frequent cause at 19 percent. VLUs take months to heal and can be challenging to resolve. According to published studies, between 18 percent and 40 percent of VLUs remain open at 6 months.23,[26],[27] Even at the lower end of that scale this represents a significant patient population and a large cost of care. In the U.S., VLUs result in treatment costs estimated at $14.8 billion and a loss of >2 million workdays annually.[28] These data illustrate the importance of effective VLU treatment protocols.
A previous study by Ma and associates[29] of a cohort of 84 patients with open C6 VLUs, who were followed for a year, detailed the immediate and short-term actual costs of treating this condition in a wound center by vascular surgeons. This study demonstrated that the mean total cost for the group was nearly $16,000 per patient/year. However, patients who did not heal their VLUs had threefold higher costs than patients who healed their VLUs without recurrence ($33,907 vs $10,563), principally due to outpatient facility fees and home nursing services. Hospitalization in a minority of patients, predominantly for infection refractory to outpatient antibiotic treatment, also led to a threefold increase in per-patient costs. These findings reinforce the importance of expediting wound healing and maximizing the ulcer-free interval.
Diagnostic Options
While diagnosing a patient with C6 disease is straightforward, diagnosing LED concurrent to CVI need not be a significant additional burden. The 2013 International Society of Lymphology consensus document[30] states, “In most patients, the diagnosis of lymphedema can be readily determined from the clinical history and physical examination.”
All chronic peripheral edema (edema persisting for more than 3 months) should be evaluated for systemic causes such as heart failure, renal failure, hypoproteinemia, and pulmonary hypertension. If the patient presents with these or other underlying conditions, they should be managed appropriately. However, causes of peripheral edema are not mutually exclusive and edema management always depends on healthy lymphatics.
To evaluate lymphatic involvement, a thorough clinical history may help to determine a potential cause of LED. An effective clinical history should include topics such as:
- Date of symptom onset
- Age of symptom onset (primary LED can develop into the fifth decade of life)
- Family history of swelling
- Time swelling occurs each day: upon waking, morning, afternoon, evening
- Whether swelling is relieved with elevation
A clinical evaluation should be performed to document signs, symptoms, and severity. For lower-extremity patients, some clinicians gather simple data points such as ankle circumference (analyzed over time) and palpation of the supramalleolar region for pitting/edema. A Kaposi-Stemmer’s Sign (inability to pinch a fold of skin in a digit in patients with LED) is also effective and extremely simple to perform.[31] While a clinician is evaluating potential venous involvement, frequently performed with duplex ultrasound (DUS), that modality is also valuable to evaluate the ultrasound characteristics of the tissue layers in the limb and monitor the responses to therapy. With this information in hand, clinicians can typically achieve an accurate LED diagnosis. For exceptional cases that may require more definitive testing, additional diagnostic tools are available.
Lymphoscintigraphy (LSG) is often referred to as the standard diagnostic test for lymphatic dysfunction and is recommended in the American Venous Forum’s 2008 guidelines[32] and in the International Society of Lymphology consensus document published in 2013.32 More recently, however, others have highlighted the many limitations of LSG. In a 2017 review and evaluation of imaging techniques to diagnose LED, O’Donnell et al.[33] cite a lack of LSG standard protocols on radionuclide usage, prolonged procedure times due to slow radionuclide uptake, and false-positive readings for early stages of LED as significant limitations. They also noted that LSG can be painful and/or uncomfortable for patients, but is nevertheless still prescribed by many as the diagnostic tool of choice to diagnose LED. The authors support DUS, commonly used to diagnose vascular dysfunction, as a readily available, non-invasive technology to visualize lymph nodes and tissue layers, provide information on the etiology and severity of LED, assess thickness of tissue segments before and after treatment, detect venous reflux, and uncover hypertrophy of connective tissue and buildup of interstitial fluid. This diagnostic technique allows researchers to confidently diagnose LED, evaluate density patterns to reveal tissue changes associated with lymphatic dysfunction, and differentiate various forms of edema.[34] DUS also correlates well with the International Society of Lymphology staging system.[35] In cases of secondary LED where a possible neoplastic etiology is suspected, CT scans are also helpful.35
Treatment Options
In the presence of CVI, treatment strategies should be modified to include early treatment to control chronic edema.32 Once an LED diagnosis is achieved, effective treatment for both venous and lymphatic conditions is often required to enable patients to return to more normal function. Fortunately, effective therapies exist for treating the lymphatic condition, and they provide vital assistance to patients and the medical community. This section will comment on treatment of the lymphatic component.
Effective treatment of LED centers on minimizing edema, improving skin condition to prevent infections as well as controlling pain and discomfort. The use of compression -- through garments such as compression stockings and compression bandaging -- is common practice to reduce venous hypertension and alleviate symptoms in patients with CVI who manifest edema, skin changes, and ulcerations.[36] Compression with mobilization also reduces capillary filtration. In a 2013 study, Dolibog et al.[37] randomized 70 patients with unilateral VLUs to compression therapy by intermittent pneumatic compression (IPC), multilayered compression systems, or short-stretch bandages for 15 days. Ulcer size reduction and percentage of wounds healed were significantly higher in the groups receiving IPC or stockings than in the groups using short-stretch bandages. However, static compression alone has not proven effective in minimizing or reversing the symptoms of LED, especially in patients with advanced stages of the disease.9
Complete decongestive therapy (CDT), including manual lymphatic drainage (MLD) massage to reduce limb volume, remains the standard of care for lymphedema patients during the acute phase of treatment.9 Patient-directed therapy is then needed to maintain limb reduction; however, compliance to self-directed therapy has historically been low and is considered the greatest obstacle to successful lymphedema treatment.[38] Use of at-home PCDs, specifically APCDs (advanced pneumatic compression devices, denoted by HCPCS Code E0652), is associated with high rates of compliance,[39] significant reductions in cellulitis rates and outpatient care,[40] hastening of VLU healing,8 and responsiveness to long-standing VLUs that resist healing with other methods.5-7 As Pearson and Mortimer stated, the ideal medical treatment for CVI would achieve both a decrease in capillary filtration and an improvement in lymphatic function.[41]
In a recent study published in the Journal of Vascular Surgery, Lerman et al.24 evaluated the impact of multiple treatment modalities on phlebolymphedema, including conservative therapy (MLD, physical therapy and compression bandaging), simple pneumatic compression devices (SPCDs; non-programmable multi-cell devices, HCPCD code E0651), and APCDs (programmable multi-cell device) on phlebolymphedema medical resource utilization and costs. The study involved a longitudinal matched case-control analysis utilizing de-identified private insurance claims information from the BMI data repository (the same population used for the above-mentioned analysis of causes and treatment of LED). The analysis demonstrated that patients treated with conservative therapy plus a specific proprietary APCD (Tactile Medical, Minneapolis, Minn.) had 69 percent lower per patient per year total phlebolymphedema and sequelae-related costs compared with patients treated with conservative therapy alone (net of any pneumatic compression device-related costs) ($3839 vs $12,253; P = 0.001). Phlebolymphedema-related costs were also significantly lower for patients treated with the specific APCD compared with patients treated with SPCDs ($1153 vs $7449; P = 0.008) and other APCDs ($3973 vs $8436; P = 0.032). Additionally, the authors found that receipt of the specific APCD was associated with 50 percent lower rates of cellulitis (22.4 percent vs 44.9 percent, P = 0.02) compared with other APCDs.
In a study41 published by investigators from New York University’s Langone Medical Center’s Division of Vascular and Endovascular Surgery, 100 patients, who were being treated for lower-extremity LED, 66 percent of whom had severe symptoms, received the specific APCD in conjunction with standard LED care that included compression, skin care and treatment for infection (if necessary). Pre- and post-treatment data were collected on the number of cellulitis episodes, presence of venous insufficiency, number of ulcers, and limb girth. A self-reported quality-of-life questionnaire was also collected before and after use of the APCD. The system was reportedly used by patients an average of 5.3 times per week for 12.7 months. All patients reported overall improvements in quality of life and LED symptoms. Among them, 89 percent considered their conditions greatly or moderately improved. Cellulitis episodes decreased by 81 percent (from 26 to 5, P = 0.002) and the number of ulcers decreased by 71percent (from 7 to 2, P = 0.007). Despite the commitment to multiple treatment sessions each week, 90 percent of patients said they would recommend the APCD to other patients.
Conclusions
Most, if not all, CVI CEAP stage 3 and greater patients have lymphatic dysfunction (phlebolymphedema), making it the leading cause of LED in the U.S. However, recognition and treatment of phlebolymphedema prior to VLU remains low. Effective treatment of phlebolymphedema is associated with improved outcomes, reduced costs and hastened the healing of VLUs. Clearly, consideration of the lymphatics should be included in modern treatment protocols for CVI and VLUs. With minor modifications to diagnosis and treatment protocols, including earlier lymphatic treatment and the prescription of effective at-home advanced pneumatic therapy devices, patients can better manage their symptoms and move toward more normal function and quality of life.
Key Points Summary:
- CVI is the leading cause of the lower-extremity LED. Recent research highlights the interdependent nature of the venous and lymphatic systems and improves our understanding of the role lymphatics play in peripheral vascular health.
- Lymphatic therapy in CVI improves outcomes, reduces the cost of care and hastens VLU healing. Therefore, lymphatic assessment and support should be standard for patients with CVI.
- To improve outcomes for CVI patients, it is imperative for health-care providers to recognize the interconnected nature of the venous and lymphatic systems, identify LED in its early stages, and address the lymphatics in parallel with the patient’s venous condition.
- In addition to the evaluation of clinical history, signs, and documented symptoms, DUS offers a viable diagnostic medium for LED.
- Advanced pneumatic compression pumps used in conjunction with standard static compression have been shown to be effective at improving outcomes and lowering costs in patients with both cancer- and non-cancer-related lymphedema, including patients with phlebolymphedema.
Citations
[1] Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560-82.
[2] Nelzén O, Bergqvist D, Lindhagen A. Leg ulcer etiology—a cross-sectional population study. J Vasc Surg. 1991;14(4):557-64.
[3] Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest. 2014;124(3):915-921.
[4] O’Meara, S., Al-Kurdi, D., Ologun, Y., Ovington, L. G., Martyn-St James, M., & Richardson, R. (2013). Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev, 12.
[5] Smith PC, Sarin S, Hasty J, Scurr JH. Sequential gradient pneumatic compression enhances venous ulcer healing: a randomized trial. Surgery. 1990;108(5):871-5.
[6] Rasmussen JC, Aldrich MB, Tan IC, et al. Lymphatic transport in patients with chronic venous insufficiency and venous leg ulcers following sequential pneumatic compression. J Vasc Surg Venous and Lymphat Disord. 2016;4(1):9-17.
[7] Farrow W. Phlebolymphedema–a common underdiagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec. 2010;2(1):14-23.
[8] White R, Ellis M, Price J, Whitaker J. Lymphovenous oedema (phlebolymphoedema): the nature and extent of the problem. Wounds UK. 2014;10(1):22-8.
[9] Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87(2):198-210.
[10] Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19(4):312-26.
[11] Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004; 557(3):889-907. doi:10.1113/jphysiol.2003.058255.
[12] Fowkes FG, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology. 2001;52:S5-S15.
[13] Partsch H, Lee BB. Phlebology and lymphology–a family affair. Phlebology. 2014;29(10) 645–647
[14] Nicolaides AN. Investigation of chronic venous insufficiency: a consensus statement. Circulation. 2000;102(20):e126-63.
[15] Scelsi R, Scelsi L, Cortinovis R, Poggi P. Morphological changes of dermal blood and lymphatic vessels in chronic venous insufficiency of the leg. Int Angiol. 1994;13(4):308-11.
[16] Ruocco E, Brunetti G, Brancaccio G, Schiavo AL. Phlebolymphedema: Disregarded cause of immunocompromised district. Clin Dermatol. 2012;30(5):541-3.
[17] Kataru RP, Baik JE, Park HJ, Wiser I, Rehal S, Shin JY, Mehrara BJ. Regulation of immune function by the lymphatic system in lymphedema. Front Immunol. 2019;10
[18] Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QjM. 2004;97(7):431-7.
[19] Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-46.
[20] Bunke N, Brown K, Bergan J. Phlebolymphemeda: usually unrecognized, often poorly treated. Perspect Vasc Surg Endovasc Ther. 2009;21(2):65-8.
[21] Lerman M, Gaebler JA, Hoy S, Izhakoff J, Gullett L, Niecko T, Karaca-Mandic P, O'Donnell T, Rockson SG. Health and economic benefits of advanced pneumatic compression devices in patients with phlebolymphedema. J. Vasc Surg. Published online: June 15, 2018.
[22] Marston WA, Carlin RE, Passman MA, Farber MA, Keagy BA. Healing rates and cost efficacy of outpatient compression treatment for leg ulcers associated with venous insufficiency. J Vasc Surg. 1999;30(3):491-8.
[23] Son A, O’Donnell TF, Izhakoff J, Niecko T, Gaebler JA, Iafrati MD. The causes of lymphedema and the associated treatment gap. J Vasc Surg Venous Lymphat Disord (In Press)
[24] Data on file with Tactile Medical
[25] Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050-8.
[26] Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol. 2000;142(5):960-4.
[27] Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363(9424):1854-9.
[28] Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347-56.
[29] Ma H, O'Donnell Jr TF, Rosen NA, Iafrati MD. The real cost of treating venous ulcers in a contemporary vascular practice. J Vasc Surg Venous Lymphat Disord. 2014;2(4):355-61
[30] The International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology. 2013;49(4):170-84.
[31] Brenner E, Putz D, Moriggl B. Stemmer's (Kaposi-Stemmer-) sign—30 years later. Phlebologie. 2007;36(6):320–324.
[32] Gloviczki et al. Handbook of Venous Disorders, Third Edition. CRC Press. 2008
[33] O'Donnell TF, Rasmussen JC, Sevick-Muraca EM. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord. 2017;5(2):261-73.
[34] Suehiro K, Morikage N, Murakami M, Yamashita O, Ueda K, Samura M, et al. Subcutaneous tissue ultrasonography in legs with dependent edema and secondary lymphedema. Ann Vasc Dis. 2014;7(1):21-7.
[35] Suehiro K, Morikage N, Murakami M, Yamashita O, Samura M, Hamano K. Significance of ultrasound examination of skin and subcutaneous tissue in secondary lower extremity lymphedema. Ann Vasc Dis. 2013;6(2):180-8.
[36] Rabe E, Partsch H, Hafner J, et al. Indications for medical compression stockings in venous and lymphatic disorders: An evidence-based consensus statement. Phlebology. 2018;33(3):163-84.
[37] Dolibog P, Franek A, Taradaj J, et al. A randomized, controlled clinical pilot study comparing three types of compression therapy to treat venous leg ulcers in patients with superficial and/or segmental deep venous reflux. Ostomy Wound Manage. 2013;59(8):22-30.
[38] Mayrovitz HN. The standard of care for lymphedema: current concepts and physiological considerations.
Lymphat Res Biol. 2009;7(2):101-8.
[39] Ridner SH, McMahon E, Dietrich MS, Hoy S. Home-based lymphedema treatment in patients with cancer-related lymphedema or noncancer-related lymphedema. Oncol Nurs Forum. 2008; 35(4):671-80.
[40] Karaca-Mandic P, Hirsch AT, Rockson SG, Ridner SH. The cutaneous, net clinical, and health economic benefits of advanced pneumatic compression devices in patients with lymphedema. JAMA Dermatol. 2015;151(11):1187-93.
[41] Pearson IC, Mortimer PS. Lymphatic function in severe chronic venous insufficiency. Phlebolymphology. 2004;44:231-67


