by Isabela T. Jones, MD, FAAD and Monique J. Vanaman Wilson, MD


Vein specialists have many options when choosing which sclerosants to use for the treatment of lower extremity reticular veins and telangiectasias. In general, the sclerosant type, potency and concentration need to be tailored to the vessel caliber in order to maximize efficacy while minimizing potential complications.
While no perfect solution exists, the use of polidocanol (POL) foam for reticular veins and glycerin for telangiectasias has demonstrated efficacy and safety in the medical literature.2-7
Sclerosant options
Glycerin, or glycerol, is a viscous compound that likely functions within the vessel as both a chemical irritant and dehydrating osmotic agent. It has relatively weak sclerosing power, making it ideal for use in telangiectasias.
Pigmentation and necrosis with glycerin are extremely rare, even after extravascular injection.8,9 Unlike POL, glycerin has not been approved by the Food and Drug Administration for sclerotherapy. Because of this, there are no standards for the way that glycerin should be used and injected, and practitioners often compound glycerin with chromium alum, lidocaine or epinephrine.
Chromium alum has been shown to prevent hematuria and enhance the sclerosing effects of glycerin.7 However, the addition of chromium has potential complications. First, there have been cases of hypersensitivity reactions with chromated glycerin.10 Second, studies have reported that chromium causes a greater incidence of ulceration, hyperpigmentation and telangiectatic matting.11
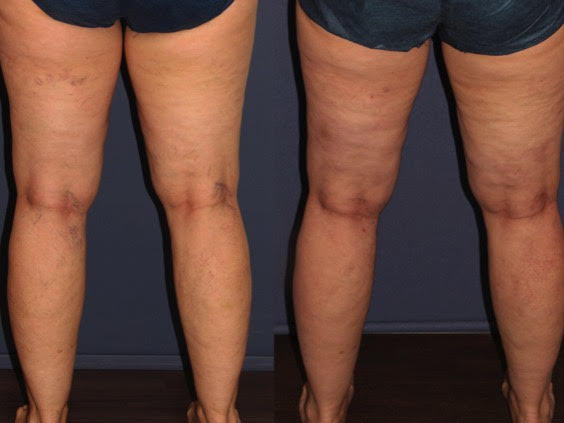
To mitigate local pain during injection and because of glycerin’s high viscosity, it is often partially diluted with lidocaine. Kern et al. demonstrated that the addition of lidocaine and epinephrine decreased the pain of glycerin injections.12
Studies on sclerosants
In their study, 110 patients were randomized to receive treatment to one thigh with either chromated 72 percent glycerin, or chromated 72 percent glycerin mixed 2:1 with one percent lidocaine with epinephrine. Both reticular and telangiectatic veins were injected with the glycerin solution. They found that treatment results were similar between both groups, with no differences in the incidence of microthrombi, telangiectatic matting and pigmentation.
Some physicians advocate the use of lidocaine with epinephrine rather than plain lidocaine when diluting glycerin.1 The hypothesis is that vasospam induced by epinephrine will allow for prolonged contact between the injected glycerin and the vessel wall, possibly increasing the efficacy of the treatment. In addition, the vasospasm could prevent the leakage of red blood cells, thereby reducing ecchymoses and pigmentation associated with injection.
Because no studies have previously examined the effect of epinephrine upon the efficacy and safety of sclerotherapy, we decided to conduct a prospective, double-blind, split-body study to investigate these hypotheses. We enrolled 30 subjects, between the ages of 30-70, who presented with bilateral, symmetric lower extremity reticular and telangiectatic veins, without the presence of varicose veins.
Once enrolled, each leg of the patient was randomized to receive one of two treatments. One leg was injected first with foamed POL for the reticular veins, followed by glycerin with lidocaine for telangiectasias. The contralateral leg was also injected with foamed POL for reticular veins, but glycerin with lidocaine and epinephrine was used for the telangiectasias.
We utilized non-chromated 99 percent glycerin, which was then diluted 2:1 with one percent lidocaine hydrochloride without epinephrine or with one percent lidocaine hydrochloride USP with epinephrine 1:100,000, resulting in a final concentration of 48 percent glycerin in both solutions. After treatment, all patients were asked to use compression stockings for two weeks.
Patients returned at days 14 and 60 for assessments. Overall, we found no differences in the degree of vessel clearance between the two treatment groups (glycerin with or without epinephrine). Both regimens produced a mean 51-75 percent improvement in reticular and telangiectatic veins, as rated by a blinded investigator. Patients tended to grade their degree of improvement slightly lower than the investigators.
Side effects (erythema, pigmentation, urtication or swelling, ankle/pedal edema, ecchymosis, ulceration, telangiectatic matting and hyperpigmentation) were graded immediately after treatment and at days 14 and 60. We found no difference in the degree of adverse events between the two groups.
The level of pain during and after the injection, pruritus, edema, erythema, bruising, ulcerations and pigmentation measured by patients daily for 14 days after treatment also did not differ significantly. The only significant difference we found was that patients reported less swelling and redness immediately after treatment in the leg that was treated with epinephrine.
The exact details of the methods and assessments we used in the study, in addition to a more in-depth analysis of our study results, can be found in the manuscript, which has been submitted for publication.
Summary
Overall, our study failed to confirm the hypothesis of previous physicians that the addition of epinephrine to the glycerin solution could increase the efficacy and safety of sclerotherapy. This underscores the importance of randomized controlled studies in testing practices that affect patient care, especially when the proposed method introduces potential complications. While not observed in our study, epinephrine can cause tachycardia, hypertension, tremor and anxiety. Caution is recommended in patients with certain medical coanditions, including severe peripheral artery disease, pheochromocytoma, hyperthyroidism, unstable cardiac disease and glaucoma. Nonselective beta blockers, tricyclic antidepressants, phenothiazides and monoamine oxidase inhibitors can increase epinephrine sensitivity.13 Our study was small and likely underpowered, and larger randomized controlled trials are needed to further evaluate the role of epinephrine in sclerotherapy.
Moving forward, there is convincing evidence that blending glycerin with lidocaine can decrease the pain of injection.10 Certainly, minimizing pain during office procedures enhances the patient experience, satisfaction, trust in the physician and likelihood of repeating and recommending the treatment. It also allows physicians to focus on the task at hand.
References
- Duffy DM. Sclerosants: a comparative review. Dermatol Surg. 2010;36:1010-1025.
- Palm MD, Guiha IC, Goldman MP. Foam sclerotherapy for reticular veins and nontruncal varicose veins of the legs: a retrospective review of outcomes and adverse effects. Dermatol Surg. 2010;36 Suppl 2:1026-33.
- Peterson JD, Goldman MP, Weiss RA, Duffy DM, Fabi SG, Weiss MA, et al. Treatment of reticular and telangiectatic leg veins: double-blind, prospective comparative trial of polidocanol and hypertonic saline. Dermatol Surg. 2012;38(8):1322-30.
- Goldman MP, Sadick NS, Weiss RA. Cutaneous necrosis, telangiectatic matting, and hyperpigmentation following sclerotherapy. Etiology, prevention, and treatment. Dermatol Surg. 1995;21(1):19-29; quiz 31-2.
- Sadick NS. Choosing the appropriate sclerosing concentration for vessel diameter. Dermol Surg. 2010;36:976-981.
- Goldman MP, Geux J-J, Weiss RA. Sclerotherapy: Treatment of Varicose and Telangiectatic leg veins. 5th ed. Philadelphia: Mosby Elsevier; 2011.
- Goldman NP. My sclerotherapy technique for telangiectasia and reticular veins. Dermatol Surg. 2010;36:1040-1045.
- Hutinel B. Esthetique dans les scleroses de varices et traitement des varicosities. Vie Med 1978; 20:1739.
- Nebot F. Quelques points tecniques sur le traitement des varicosities et des telangectasies. Phlebologie
1968;21:133. - Munavalli GS, Weiss RA. Complications of sclerotherapy. In: Seminars in cutaneous medicine and surgery. Philadelphia, PA: WB Saunders; 2007; pp. 22–8.
- Ghaznavi Am, Nakamura M, Tepper AD. An Analysis of 72% chromated glycerin used for sclerotherapy: sterility, potency, and cost after extended shelf life. Dermatol Surg. 2015 Jan;41(1):121-5.
- Kern P, Ramelet AA, Wutschert R, Mazzolai L. A double-blinded, randomized study comparing pure chromated glycerin with chromated glycerin with 1% lidocaine and epinephrine for sclerotherapy of telangiectasias and reticular veins. Dermatol Surg. 2011;37(11):1590-1594.
- Kouba DJ, LoPiccolo MC, Alam M, Bordeaux JS, Cohen B, Hanke CW, Jellinek N, Maibach HI, Tanner JW, Vashi
N, Gross KG, Adamson T, Begolka WS, Moyano JV. Guidelines for the use of local anesthesia in officebased
dermatologic surgery. J Am Acad Dermatol. 2016 Jun;74(6):1201-19.


